Introduction
The processing of meat is infamous for producing large amounts of byproducts, such as blood, bones, meat trimmings, skin, fatty tissues, horns, hoofs, feet, viscera, and skin (Toldrá et al., 2016). Concerns regarding these byproducts’ environmental effects arise because it can be challenging and expensive to dispose of them in an environmentally friendly way. Furthermore, properly handling and disposal of these byproducts can significantly raise the overall cost of the production process (Ryder et al., 2015). Even though some byproducts of the meat industry may be difficult to dispose of, they may also be very nutritious and used in foods worldwide (Rahman et al., 2014). Byproducts from several organs, including the liver, lung, heart, kidney, brain, spleen, and tripe, are often used in conventional dishes across many cultures (Nollet and Toldra, 2011). These byproducts are essential for constructing nutrient-dense meals since they frequently include high protein levels, vitamins, and minerals (Soladoye et al., 2022). Utilizing these byproducts in specific circumstances can also help prevent food waste and promote more sustainable food systems (Jurgilevich et al., 2016).
Meat byproducts can be used in various ways, including as ingredients in animal, poultry, and aquatic animal feed and in the manufacturing of pet foods (Thompson, 2008). They can also be used as a source of novel materials that can replace plastics and biodiesel manufacturing. Meat byproducts can also be converted into bioactive peptides, which have potent physiological effects, or protein hydrolysates, which have a variety of technical uses (Toldrá et al., 2016). Many cultures are aware of the nutritional composition of meat byproducts. They utilized them in their diet as an excellent source of major amino acids, minerals, fats, and proteins (Alao et al., 2017).
The liver is also a great source of vitamins, particularly vitamins A, B, C, and D. The liver can be ingested directly in raw form or even in well-processed form, depending on the individual’s preferences (Alao et al., 2017). Many sectors are currently utilizing beef liver to facilitate the protein content of their food items. It is also being incorporated in complementary feeds to provide proper nutrition to children and save them from protein energy malnutrition (Ryckman et al., 2021). Chicken liver is also nutritious and eaten worldwide. Due to its nutritional profile, it is added as a protein replacement in processed items like sausages (Choe et al., 2019). This research article showed the formulation of chicken nuggets supplemented with beef and chicken livers to enhance the overall nutritional profile of the nuggets.
Materials and Methods
Liver samples were collected from the local market in Johar town, Lahore, Pakistan, transported under cold conditions, and stored at 4°C in sealed bags. Analytical-grade chemicals were used for all experiments.
Compositional analysis (moisture, ash, fat, and protein) was performed using the AOAC method (930.15, 942.05, 920.39, and 984.13, respectively). The compositional profile of beef and chicken livers was measured on alternate days (days 0, 1, 3, 5, and 7) to understand the degradation rate.
The oxidative stability of fat was determined through free fatty acids (FFA) and peroxide value (POV) by following the method of Akhter et al. (2022). For FFA, a 5 g minced sample was dissolved in 30 mL of chloroform, mixed at 10,000 rpm for 1 min with a homogenizer, and filtered using Whatman filter paper no. 1 to remove particles. Then, 5 drops of 1% ethanolic phenolphthalein were added, followed by titration with 0.01 or 0.1 N ethanolic potassium hydroxide, depending on fat content. For POV, a 3 g minced sample was melted at 60°C for 3 min in an Erlenmeyer flask. Then, 30 mL of 3:2 (v/v) acetic acid and chloroform mixture was vigorously mixed for 3 min. After filtration with Whatman filter paper no. 1, 0.5 mL of saturated potassium iodide and 0.5 mL of 1% starch solution were added, followed by titration with 0.01 N sodium thiosulfate.
The antioxidant potential was determined with the help of two analyses, which included the radical scavenging potential accessed through 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay as per the method of Verma et al. (2017). The samples were evaluated at 4°C temperature. Twenty-five μL of the homogenized sample was mixed with 1 mL of prepared DPPH solution and 0.25 mL of Tris-HCl buffer. The absorbance was measured at 517 nm. Whereas, the antioxidant potential present due to phenolic content was determined through total phenolic content (TPC) according to the methodology described by Wong-Paz et al. (2015). The homogenized sample (0.5 mL) was mixed with 10% Folin-Ciocalteu reagent (2.5 mL), followed by the addition of 7.5% sodium carbonate (2.5 mL). After 45 min incubation at 45°C, absorbance at 765 nm was measured and compared to a gallic acid reference curve.
Beef and chicken liver were nutritionally profiled with the proximate analyses. Then, each quantity was added to chicken nuggets to improve their nutritional value. The experiment was set up so that there was a single positive and negative control, with the negative control having no value addition, as indicated in Table 1. Only the positive control contained texturized soy protein (TSP), which assisted in comparing the nutritional value of nuggets with and without the TSP and the value addition of the liver. The nuggets were then prepared using the standard recipe for all the formulations, as given in Table 1.
The color analysis of CIE L*, CIE a*, CIE b* color values defining CIE L*, hue, and saturation of beef and chicken livers were measured according to the method described by Abd-El-Aziz et al. (2022) with the help of a standardized colorimeter (CR-20, Konika Minolta, Tokyo, Japan). All the readings were taken in triplicates.
Texture profiling of the nuggets was performed using Imada Texture Analyzer. The treatments’ nuggets were fried at 130°C for 4–5 min in an electric deep fryer and were placed on the texture analyzer surface. A suitable probe (diameter=20 mm) was used to check the nuggets’ hardness, cohesiveness, springiness, and chewiness. The compression speed was kept at 2 mm per sec, whereas the displacement for compression and returning speed were kept at 5 mm per sec.
A descriptive sensory evaluation based on a hedonic preference test was done to assess the acceptability of two groups of liver-supplemented nuggets, for which a panel was formed after the taste testing. Each panelist was instructed to use a 9-point hedonic scale to score the samples for color, texture, taste, flavor, and overall acceptability. Water was provided to the panelists so they could rinse their mouths between samples.
The collected data was processed through statistical analysis using analysis of variance (ANOVA) based on the Completely Randomized Design method. The significance of differences between means was determined at a 95% probability level threshold. The results were reported as mean±SD.
Results and Discussion
A significant decrease was observed (p<0.05) in all the compositional parameters over the storage period of 7 days. Beef liver (Table 2) has a better nutritional profile and stability over 7 days than the chicken liver. The shelf stability of beef and chicken liver was estimated with the help of first-order kinetics, which explained the protein degradation rate concerning storage time in days. The protein degradation kinetics differed significantly (p<0.05) between the two types of livers, chicken and beef. The half-life values (Fig. 1) at 4°C were >16 days for chicken liver and >23 days for beef liver.
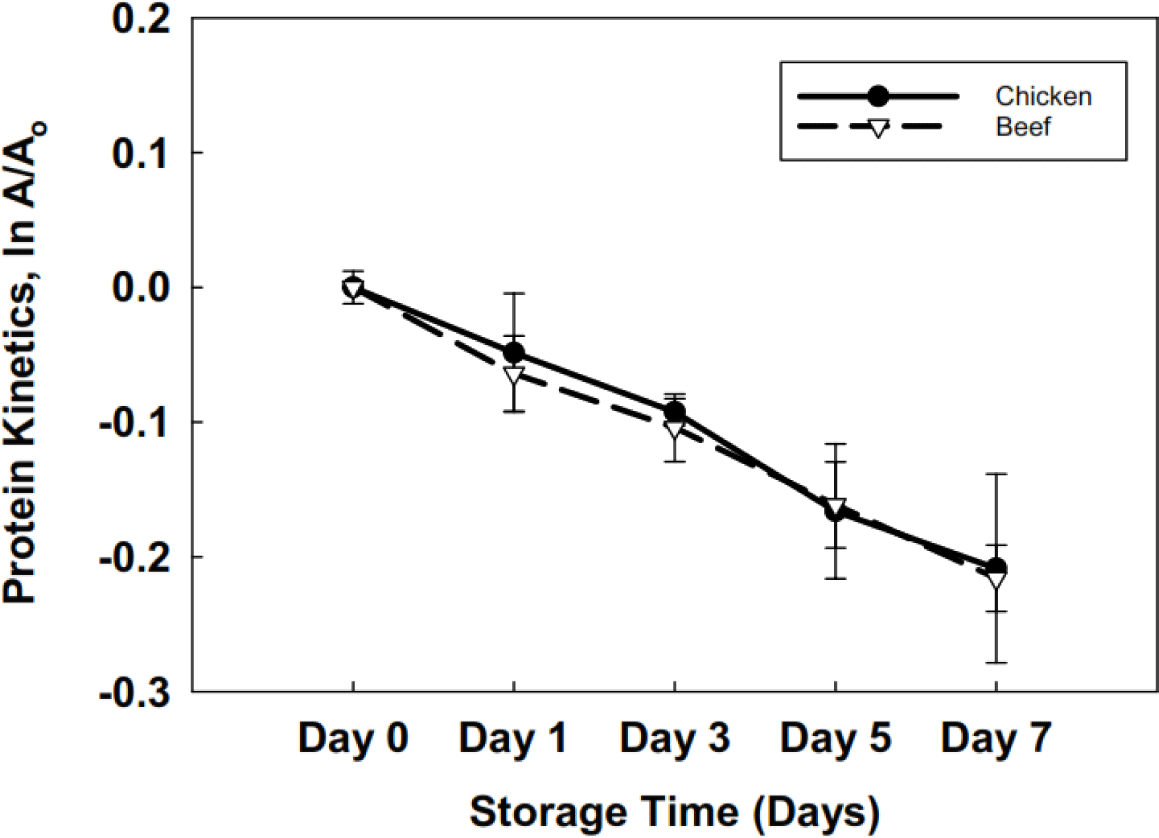
Comparable moisture content findings of approximately 74.9% for beef liver were reported by Kakimov et al. (2018). The decline in moisture and ash content can be attributed to a reduction in water-holding capacity (Hughes et al., 2014). Temperature changes, particularly transitioning from a refrigerated environment to room temperature and handling practices, can contribute to decreased ash content in broiler meat (Augustyńska-Prejsnar et al., 2019). The degradation of meat lipids can be attributed to the intermediate actions of endogenous meat enzymes, leading to fat hydrolysis (Agnihotri, 1988). The disparity in fat percentage between beef and chicken liver may be attributed to the higher antioxidant potential of beef liver, although no specific study on the antioxidant potential of beef liver exists. The possible reason for protein degradation might be the oxidation of proteins when exposed to the environment; moreover, endogenous enzymes’ enzymatic activity can cause protein degradation (Akhter et al., 2022; Lan et al., 2022).
The statistical analysis showed a significant (p<0.05) increase in the compositional content (moisture, ash, fat, and protein) of nuggets after the addition of different liver concentrations, as indicated in Table 3. The ash content varied between 1.9% to 2.0% with the addition of different concentrations of beef liver. In comparison, adding chicken liver increased the ash contents in nuggets from 1.7% to 1.8%. Each treatment markedly increased the fat present; however, the latter two had comparable fat levels. While beef liver-treated nuggets had 16.5% to 20.4% fat, control nuggets had 12.6% to 13.3% fat. Beef liver contributed more to the fat content of chicken liver-based nuggets, which had a 14.1% to 15.5% fat content.
Positive control (12%) outperformed the negative control (11.9%) in protein content, attributed to soy inclusion (Yuan et al., 2021). Beef liver-treated nuggets had 11.8% to 13.0% protein, and chicken liver-treated nuggets contained 11.3% to 12.5%, showing that addition of liver content from 5% to over 10% boosted protein content more than controls.
Using chicken liver resulted in higher moisture content in chicken liver-based pate (Porto-Fett et al., 2019). In their study, the moisture content was recorded to be 74.2%. On the other hand, an increasing trend in moisture content was observed by Devatkal et al. (2004), where the moisture content of the end food product increased from 68% to 69% with the addition of beef liver in the meat loaves. The liver is a rich source of micronutrients (Jurgilevich et al., 2016). One of the studies reported an increase in total ash or mineral content by adding chicken liver to sausages. El-Sayed et al. (2018) reported an increase from 3.4% to 7.7%, from 0% to 20% of chicken liver addition, respectively. This justifies the ameliorating nutritional content of formulated chicken nuggets with beef and chicken liver supplementation.
Adding the liver increases the fat content as it was also estimated by adding the liver in liver pate (Estévez et al., 2005). Meat byproducts, predominantly liver, can increase the food item’s overall fat and protein content (Bujak, 2015). Adding chicken liver meat to sausages also increased the overall protein content of the sausages from 34.6% to 37.9% (El-Sayed et al., 2018). The same results were observed when beef liver in powdered form was added to cakes and cookies, resulting in increased protein content and increased beef liver powder concentration (Folorunso and Ayooluwa, 2021). Thus, these studies validate the increase in the nutritional profile of chicken nuggets with beef and chicken liver supplementation.
A significant increase (p<0.05) was observed in both POV and FFA values of both livers over the storage period of 7 days, as shown in Figs. 2A and B, respectively. Whereas, while talking about the value-added nuggets, a significant divergence was observed regarding the increment of POV and FFA values, as shown in Figs. 2C and D, respectively. The increase might be due to the susceptibility of the liver to oxidation, and the presence of polyunsaturated fatty acids increases the susceptibility to peroxidation (Wąsowicz et al., 2004).

The antioxidant potential of the livers decreased significantly (p<0.05) with respect to the storage time, as shown in Figs. 3A and B. The radical scavenging activity of the liver-based nuggets increased significantly (p<0.05) with respect to increasing liver concentrations, as shown in Figs. 3C and D. This heightened antioxidant potential may be attributed to elevated phenolic content and stable feeding practices. However, research on storage effects and antioxidant enhancement in animal liver and liver-based products is ongoing (Wang et al., 2017). TPC significantly (p<0.05) increased with rising liver concentration. Control samples had lower TPC (152.0% positive, 164.3% negative) than liver-added nuggets, which exhibited increasing TPC values with higher liver content.

The liver having more fat is more susceptible to oxidation and fat degradation, resulting in the instability of fatty acid contents. A similar trend was reported by Akhter et al. (2022) while conducting the same analysis on the beef liver, where they concluded that altered ratios between saturated and unsaturated fatty acids are considered unfavorable from a dietary perspective.
It is worth noting that polyunsaturated fatty acids increase susceptibility to peroxidation, thereby contributing to undesirable odors (Wąsowicz et al., 2004). POV and FFA trend reported by Akhter et al. (2022) for beef liver gives a brief idea of this increasing trend. However, no specific studies are present in this context to support the particular trend regarding processed food items.
The beef liver exhibited strong but unstable antioxidant potential compared to chicken liver, likely due to rich phenolic content and consistent feeding practices. However, no storage-related research on animal liver inhibition activity exists, and ongoing studies aim to enhance meat and liver antioxidant potential through supplementation (Wang et al., 2017).
The decline in liver antioxidant potential could be due to environmental exposure, promoting radical oxidation and formation (Echegaray et al., 2021; Islam et al., 2015), a topic lacking prior research. The liver has a high antioxidant potential that leads to oxidative stability, as a study on porcine liver-extracted hydrolysates showed a high scavenging potential of free radicals (Verma et al., 2017). Literature also suggests using pomegranate peel-based coatings (Bashir et al., 2022) and frozen white cauliflower (El-Anany et al., 2020) to boost antioxidant activity in chicken nuggets. This antioxidant potential enhancement by the liver can be seen in the chicken nuggets supplemented with liver.
Color is also one of the main quality parameters that is observed visually with the help of a colorimeter. The color evaluation was determined to evaluate the color change in nuggets due to adding livers. A significant increase (p=0.001) in CIE L* (brightness/darkness) values was observed; however, CIE a* (redness/greenness) and CIE b* (yellowness/blueness) showed minimal or no changes. The overall color change index △E* with respect to different treatments of livers was also calculated using the formula reported by Ghorbani et al. (2021).
The results revealed that the color changes were perceptible to human detection (Delta E range between 1–2) when observed closely, as slight change was observed with respect to the control. However, the values of liver-supplemented nuggets lie between Delta E values 5–8, as shown in Table 4, which revealed that these values are perceptible at a glance (Minaker et al., 2021). As the addition of liver was done through manual mixing, it made the liver somewhat visible, leaving an impact on the overall color properties of the nuggets. However, the treated nuggets were not much different from each other.
Texture analysis of chicken and beef liver-based chicken nuggets revealed significant differences (p<0.05) among all treatments and the control, with notable variations in hardness, cohesiveness, gumminess, springiness, and chewiness, as shown in Table 5. However, the same results were observed for all the parameters in chicken nuggets supplemented with chicken and beef liver. Among the treatments of chicken nuggets supplemented with chicken and beef liver, T1 exhibited the highest hardness, while T3 had the lowest hardness and chewiness, likely attributed to its higher moisture content due to increased liver concentration. Trends in cohesiveness, hardness, and springiness were comparable across chicken and beef liver nuggets. Beef liver nuggets displayed superior chewiness and gumminess.
The texture is important in determining the quality and defining the major characteristics (Yuan et al., 2021). Under this wider texture domain, hardness or tenderness is important as it determines consumer acceptability. Hardness indicates protein texturization after formulating the final product (Samard and Ryu, 2019). Gumminess and cohesiveness increased with higher liver concentration, enhancing ingredient interlocking and binding capacity. The same results were found when goat patties were formulated with full-fat soya paste (Biswas et al., 2011).
Thus, it was observed that the addition of liver does impact the overall texture profile of chicken nuggets in a positive context. The springiness, gumminess, and chewiness of meat and liver-based loaves showed similar results, and a decreasing trend from control to liver-based loaves paralleled our defined results (Devatkal et al., 2004).
The sensory evaluation of the formulated value-added chicken nuggets was performed to determine the consumer perception and acceptability regarding the addition of liver. The sensory criteria have scored under an acceptable level for all kinds of treatments. Additionally, there was no difference between the different treatments for cooked and uncooked products, as shown in Fig. 4. T1 achieved the highest overall acceptability in sensory evaluation for chicken liver-based nuggets, while T3 had the lowest scores across various parameters. T2 fell between these extremes, indicating that adding chicken liver improved consumer acceptability compared to the control, as shown in Fig. 5.


In the sensory evaluation of beef liver-based chicken nuggets, T1 had the highest overall acceptability with favorable scores across parameters. T2 and T3 had similar, lower acceptability, likely due to intensified beef liver taste and smell as concentration increased, as shown in Fig. 5.
The results obtained from sensory analysis were further statistically analyzed by Kruskal-Wallis H, and the mean score was reported for each treatment at a 95% probability level. The mean score of parameters for chicken liver-supplemented nuggets showed no significant difference between the control nuggets and all the other treatment samples, other than the overall appearance, which was statistically different (p=0.032) for all the treatments. However, beef liver-supplemented nuggets showed all the parameters to be statistically the same (p<0.05). This exhibited that the nuggets were near to the control samples and had the potential to be liked by the consumers.
The sensory evaluation results depicted the likeliness of consuming beef and chicken livers in processed food items. Beef liver-based hamburger with oats has great acceptability between children and adults, showing the potential likeliness of beef liver and the capacity to be added to processed food items (Rocha et al., 2018). Similarly, the beef liver showed acceptable organoleptic profiling regarding liver meat pate (Kolbábek et al., 2019). This illustrates the market and consumer acceptability of beef and chicken liver, as many products are already being evaluated with beef and chicken liver.
Conclusion
As a competitive substitute for texturized vegetable protein (TVP) in the consumer market, optimizing the use of chicken and beef liver presents a promising path for improving value, palatability, and formulation cost efficiency. This study investigates the incorporation of chicken and beef liver in processed foods, looking at antioxidants, proximate variables, and shelf stability over seven days. However, during preservation, the nutritional value and stability of the liver drastically decrease. Although the liver is known to be a nutrient-rich source, its stability difficulties point to the potential for incorporating the liver as a functional ingredient in innovative cuisines. Improved nutritional and organoleptic qualities are revealed when the chicken nuggets enhanced with chicken and beef liver are evaluated. These products replace texturized vegetable protein and greatly enhance protein content. They also contain more water, ash, fat, and protein and have better antioxidant properties. These products are a healthy alternative for consumers, considering the antioxidants in the liver. Positive results from texture analysis and sensory evaluation demonstrate the foods’ suitability for consumption and acceptability. Liver, which is frequently regarded as waste, has significant nutritional potential and may one day improve the nutrition of processed meat products and aid in achieving sustainable development objectives.













