Introduction
Pork is the most widely consumed meat globally, accounting for approximately one-third of total meat consumption and it is highly favored by consumers because abundant nutritional value and excellent sensory quality (OECD and FAO, 2023). During processing, storage, and marketing, microbial contamination is a major factor in pork quality deterioration and can accelerate protein and lipid oxidation, shortens shelf life, and lead to economic losses and food safety concerns (Zhou et al., 2024). Packaging serves as an effective barrier to prevent food contamination caused by physical, chemical, biochemical, and other factors (Packialakshmi et al., 2023). Simultaneously, the development and assessment of packaging materials with antibacterial properties have emerged as a current research focus and garnered significant public attention.
Chilled meat preservation has utilized a wide range of antimicrobial agents, including enzymes, polymers, organic acids, and titanium dioxide (TiO2; Dirpan et al., 2023). TiO2 have garnered significant attention as a promising antimicrobial coating in recent years (Widyastuti et al., 2023). Due to the photocatalytic activity of TiO2, it can generate reactive oxygen species under ultraviolet light exposure, which leads to microbial death by damaging cell membranes, oxidizing cellular components, or disrupting electron transfer between cell membranes (Kodithuwakku et al., 2022; Mesgari et al., 2021). Luo et al. (2015) used TiO2/low-density polyethylene composite film to preserve shrimp freshness and found that it effectively inhibited rot bacteria growth and extended the shelf life of shrimp by 8 d when stored at 4°C. Alizadeh-Sani et al. (2020) utilized whey protein isolate as the substrate to fabricate a composite film by incorporating nano-TiO2 which extended mutton's shelf life from 6 to 15 d at 4°C, with remarkable inhibition of microbial proliferation, lipid oxidation, and lipolysis in mutton. Hu et al. (2023) demonstrated incorporating 3% (w/w) TiO2 in soybean protein-based composite film exhibited significant antimicrobial activity against Bacillus cereus and Escherichia coli, effectively inhibiting their growth on the membrane surface (Chatkitanan and Harnkarnsujarit, 2020). However, the commercial application of bio-antibacterial activity packaging in meat preservation has been limited due to inherent characteristics such as high water absorption and decomposition rate, and poor barrier properties. Currently, there is a dearth of research available on the assessment of the impact of commercial plastic packaging materials containing TiO2 on meat preservation.
The present study employed air packaging (AP) and vacuum packaging (VP) as control groups to investigate the impact of vacuum antibacterial packaging (VAP) on chilled pork freshness preservation during storage. Total viable count (TVC), pH, total volatile basic nitrogen (TVB-N), sensory attributes (color, texture), and water holding capacity were analyzed to assess quality changes during storage. The study expands potential TiO2 applications in pork preservation and provides data support for developing commercial antibacterial composite films incorporating TiO2.
Materials and Methods
M. Longissimus thoracis et lumborum (LTL) muscle of six pigs (Duroc×Landrace×Yorkshire pig, 6.5 months old, 85 to 90 kg live weight) were purchased from Ershang Meat Food Group (Beijing, China). The trays and cover films for AP materials were obtained from Linhua Plastic (Ningbo, China) and Nantong Global Plastic Engineering (Nantong, China). VP was provided by Sunrise Material (Jiangyin, China). The detailed parameters are presented in Table 1. The packaging material of VAP group was prepared by co-extrusion method, and the substrate was PE/EVOH/PE. TiO2 was added to the single layer PE film with a mass fraction of 3%. Plate count agar (pH 7.0±0.2) was bought from Landbridge Technology (Beijing, China). Hydrochloric acid (HCl; 0.01 mol/L) was achieved from Regen Biotechnology (Beijing, China). Methyl red (MW: 269.3) was purchased from Yuanye Bio-Technology (Shanghai, China).
After slaughter, pork carcasses were refrigerated for approximately 24 h between 0°C–4°C before sampling. The LTL muscles were removed from six carcasses, placed in aseptic sampling bags immediately, and transported back to the lab under refrigerated conditions. Each LTL muscle was evenly divided into 15 pieces, and 78 meat samples were used in the study. The mass of each cuboid meat sample in the test ranges from 80 to 90 g. On the same carcass, six pieces of meat were randomly selected and packaged in the same treatment. The study was designed with three treatment groups (AP, VP, and VAP groups) and six storage periods (0, 3, 6, 9, and 12 d) at 4°C. Six pieces (all from different carcasses) of each treatment were measured.
VP conditions: pressure 0.74 MPa, vacuum time 20 s, heat sealing time 2 s, cooling time 3 s. After cutting, bag making, and UV irradiation for 12 h, the ordinary film and TiO2 antibacterial film were used for the VP of pork.
The TVC analysis was analyzed according to the method described in Chinese standard GB 4789.2-2022. 5 g of pork was added into a sterile bag containing 45 mL of sterile normal saline, and then homogenized and patted for 2 min to obtain a tenfold diluted sample solution. Each time, 1 mL of sample solution was sucked and added to 9 mL of sterile normal saline for ten times dilution. Three suitable dilution gradients were selected, and then 100 μL of the above sample solution was sucked and coated on the plates. Finally, all plates were incubated at 37°C for 48 h to count.
The pH value of each meat sample was measured by inserting a hand-held portable pH meter (Testo 205, Testo, Lenzkirch, Germany) into about 1.5 cm depth. Pork color was detected using a Colorimeter (CM-600d, Konica Minolta, Tokyo, Japan). Before measurement, the color difference meter needs to be calibrated. The CIE L*, CIE a*, and CIE b* values of the meat sample were recorded.
The TVB-N was detected by taking the third method in national standard of China (GB 5009.228-2016). Meat sample (5 g) was mixed in ultra-pure water (25 mL) and soaked fully for 30 min before filtration. Water-soluble glue was applied to the edge of the diffuser at first. 1 mL boric acid and a drop of mixed indicator were added to the central inner chamber of the microdiffusion dish. 1 mL filtrate and 1 mL saturated potassium carbonate solution were injected into the outer chamber. After the glass lids were covered, the microdiffusion dishes were shaken through a circular motion. All dishes were incubated at 37°C for 2 h in an incubator. Finally, the reaction solution in the center of the dish was titrated with a standard titration solution of HCl (0.01 mol/L). The mixed indicator was prepared with methyl red and bromocresol green according to a volume ratio of 1 to 5. The color of the endpoint of the titration is purple-red. The TVB-N value was expressed as mg/100 g sample.
Where: V1 and V2 is the volume of sample and blank group solution consumed HCl solution (mL); c is the strength of HCl solution (mol/L); and m is the mass of sample (g).
Before cooking, the weight of the meat sample was recorded as m1. Subsequently, the meat sample (20–30 g) was placed in the cooking bag without air, and was heated at 80°C for 20 min. After cooking, all samples were placed under cold running water to cool for 30 min. The weight of the meat sample after drying the surface moisture was represented as m2.
The moisture composition of meat samples was determined by a hydrogen proton Nuclear Magnetic Resonance Imaging (NMI; NMI20-040H-I, NIUMAG, Suzhou, China). The meat sample was cut into about 1 cm×1 cm×2 cm cubes with a flat and vertical section. Transverse relaxation time (T2) was measured with CPMG sequence. Test conditions: proton resonance frequency SF=20 MHz, 90° pulse time is 10.00 μs, 180° pulse time is 19.52 μs, repeat sampling NS=4, repetition interval TW=1,500.00 ms, number of echoes NECH=3,000, and repeat sampling frequency SW=100 kHz.
The samples were divided into 1 cm×1 cm×1 cm cubes to determine the texture properties. The cut cubes were measured by using the texture test analyzer (TA-XT plus®, Stable Micro System, London, UK). The P/50 probe was selected in the procedure, and each meat sample was measured three times. The measurement conditions were as follows: the rate before measurement was 2 mm/s, the rate during measurement was 5 mm/s, the rate after measurement was 2 mm/s, the measurement time was 5 s, the trigger force was 5 g, and the recovery height of the probe was 30 cm.
To evaluate effects of different packaging methods and storage time on TVC, pH, color, TVB-N, texture, and water holding capacity, bidirectional ANOVA was performed using SPSS 27.0 (IBM, Armonk, NY, USA). Least significant difference tests determined significance of differences (p<0.05).
Results and Discussion
The TVC is an essential parameter of reflecting the meat preservation. As displayed in Fig. 1A, the TVC of pork in AP, VP, and VAP showed an upward trend to varying degrees during storage. The TVC of pork on day 0 was 3.41 Log CFU/g. From 0 to 3 d of storage, the TVC in the three treatment groups increased slowly, and these results were consistent with Ammor et al. (2006) and Heir et al. (2022). During this period, the growth rate of the TVC in the AP group maintained a higher level. This variation might be due to the difference in oxygen content of the two packaging methods that inhibited the growth and reproduction of aerobic microorganisms (McSharry et al., 2020). During 6–12 d of storage period, the TVC in AP pork was always significantly higher than that in VP and VAP (p<0.05). Moreover, the TVC of pork in AP, VP, and VAP on day 12 was respectively 6.95, 5.93, and 5.85 Log CFU/g. The critical value of microbial of pork spoilage is 6.00 Log CFU/g according to the Chinese hygiene standard known as "Chilled pork" (NY/T 632-2002). Compared to the AP group, both VP and VAP groups can effectually extended the shelf life of chilled pork. Lu et al. (2016) showed high-barrier vacuum shrink packaging significantly extended the shelf life of pork by 3 d compared to AP group (p<0.05), consistent with this study. During storage, no significant TVC difference was observed between VP and VAP (p>0.05). The disparity was likely due to weak light intensity in the refrigerator required to stimulate TiO2 to produce reactive oxygen species, with low content produced by excitation (Wang et al., 2022). The results of transcriptome analysis demonstrated that the photocatalytic TiO2 can synergistically exert a preservation effect by significantly inhibiting cell autoregulation and membrane wall system repair, downregulating spoilage-related gene expression, and inhibit the growth of microorganisms (Yan et al., 2024). The TiO2 particles distributed on the surface of the composite film prepared by extrusion and blowing film method are relatively small, and cannot fully contact with surface bacteria directly, which inhibits the antibacterial effect of TiO2 to a certain extent (Bodaghi et al., 2013).

TVB-N value is one of main indicators for evaluating meat freshness. TVB-N refers to the enzymatic and bacterial activity in meat that facilitates protein decomposition, resulting in the production of ammonia, amines, and other nitrogenous compounds (Wang et al., 2023). In this study, the trace diffusion method was used to determine TVB-N in pork during storage, and the results are illustrated in Fig. 1B. Overall, the values in all groups exhibited an upward trend at different rates. On 0 d, the TVB-N value of pork was 2.41 units, explaining that the experimental pork had good freshness. In addition, the original values of fresh pork in other studies were 1.96 and 4.36 mg/100 g, both at a low level (Bassey et al., 2024). After 12 d during storage, the result was 18.73, 16.10 and 14.70 mg/100 g in AP, VP and VAP groups, respectively. Compared to the AP group, the data were lower in the other two groups, indicating that VP could alleviate microbial growth and endogenous protease activity, inhibiting the increase in TVB-N value caused by protein decomposition (An et al., 2023). In addition, the increase in the VAP group was significantly suppressed during 3–12 d of storage (p<0.05). The antibacterial film with antibacterial activity could efficiently improve the preservation effect of pork and delay the increase of TVB-N value (Alizadeh-Sani et al., 2020). The photocatalytic process involving TiO2 generates free radicals that induce cell death by significantly disrupting cell permeability and destroying the structure of the cell wall. Additionally, these free radicals inhibit the decomposition of proteins and other nitrogen-containing substances by microorganisms in pork. Specifically, the results of (Sheng et al., 2018) showed that the rise in TVB-N value of beef was put down to the growth of microorganism. Additionally, beef proteins are gradually degraded by bacterial contamination, namely Pseudomonas and Lactobacillus, within 12 d of storage (Bekhit et al., 2021). Therefore, the greater microorganisms multiplication, the higher the meat spoilage.
pH value is a crucial index reflecting quality changes of chilled pork. Generally, pH the increase is due to production of alkaline autolytic compounds, nitrogenous compounds, and accumulation of bacterial metabolites from protein breakdown and microbial proliferation (Pabast et al., 2018). Table 2 shows the effects of different packaging methods and materials on chilled pork pH during storage. The original pH value was 5.61 on 0 d. While pork pH showed increase in all treatments, a rapid rise occurred from 0 to 6 d, followed by slower growth from 6th d onwards. Protein decomposition and alkaline substance accumulation largely caused pork spoilage. Cheylebacterium and Serratia could cause early mutton deterioration under VP (Rood et al., 2022). A large amount of acidic substances produced by anaerobic microorganisms and alkaline substances produced by protein decomposition in the three treatment groups at the late storage stage may have been neutralized, slowing the pH value increase rate from 6 to 12 d of storage. The photocatalytic activity of TiO2 generates numerous free radicals that interact with intracellular DNA, leading to the disruption of its molecular structure and causing metabolic disorders within cells, which results in a decrease in the pH value of bacterial suspension. Studies have shown that the number of Lactobacillus in vacuum-packed pork increases rapidly during 10–20 d of storage in a refrigerated environment (Yang et al., 2023). Lactobacillus utilize carbohydrates and produce related compounds such as acetourea and diacetyl, which have unpleasant odors (Kandler, 1983). In addition, the increase in the number of Lactobacillus in vacuum-packaged dear meat at the later stage of storage led to the production of lactic acid and acetic acid, which resulted in the decrease of pH value (Sauvala et al., 2023). As a result, there was no striking difference in the pH change of pork among the treatment groups throughout storage, which was also in line with Gu et al. (2023).
| Storage time (d) | AP | VP | VAP |
|---|---|---|---|
| 0 | 5.61±0.03 | 5.61±0.03 | 5.61±0.03 |
| 3 | 5.71±0.07 | 5.75±0.07 | 5.73±0.07 |
| 6 | 5.87±0.09 | 5.84±0.11 | 5.81±0.05 |
| 9 | 5.90±0.07 | 5.87±0.10 | 5.84±0.07 |
| 12 | 5.93±0.14 | 5.90±0.09 | 5.82±0.06 |
Table 3 shows the changes of chilled pork color in different packaging methods and materials during storage at 4°C. Meat color impacts purchasing choices as it represents quality changes (Mancini and Hunt, 2005). CIE L* and CIE a* are key indicators of consumer perception and selection. Generally, meat with higher CIE a* value is more aesthetically pleasing, while meat with lower CIE L* value appears less fresh and darker (Suman et al., 2014). After 3–6 d of storage, the CIE L* value of pork in the AP group exhibited a significantly higher level compared with the VP and VAP groups (p<0.05). According to Zhang et al. (2023), the increase in CIE L* value may be attributed to two aspects. On the one hand, endogenous enzymes contribute to changes in meat microstructure, surface light scattering, and initial myoglobin oxygenation; on the other hand, rising free water content increases the meat light scattering coefficient. After 12 d of storage, pork in VAP group demonstrated a statistically significant increase in CIE a* value (p<0.05). The reported findings suggest that TiO2 particles exhibit certain antioxidant properties (Alizadeh-Sani et al., 2018). Consequently, myoglobin in the VAP group pork remains oxygenated, thereby inhibiting the formation of ferrimyoglobin. This probably indicating that VP is an effective means of preventing myoglobin oxidation.
A,B In the same column, different capital letters indicate a significant difference (p<0.05) between the treatments at the same time point.
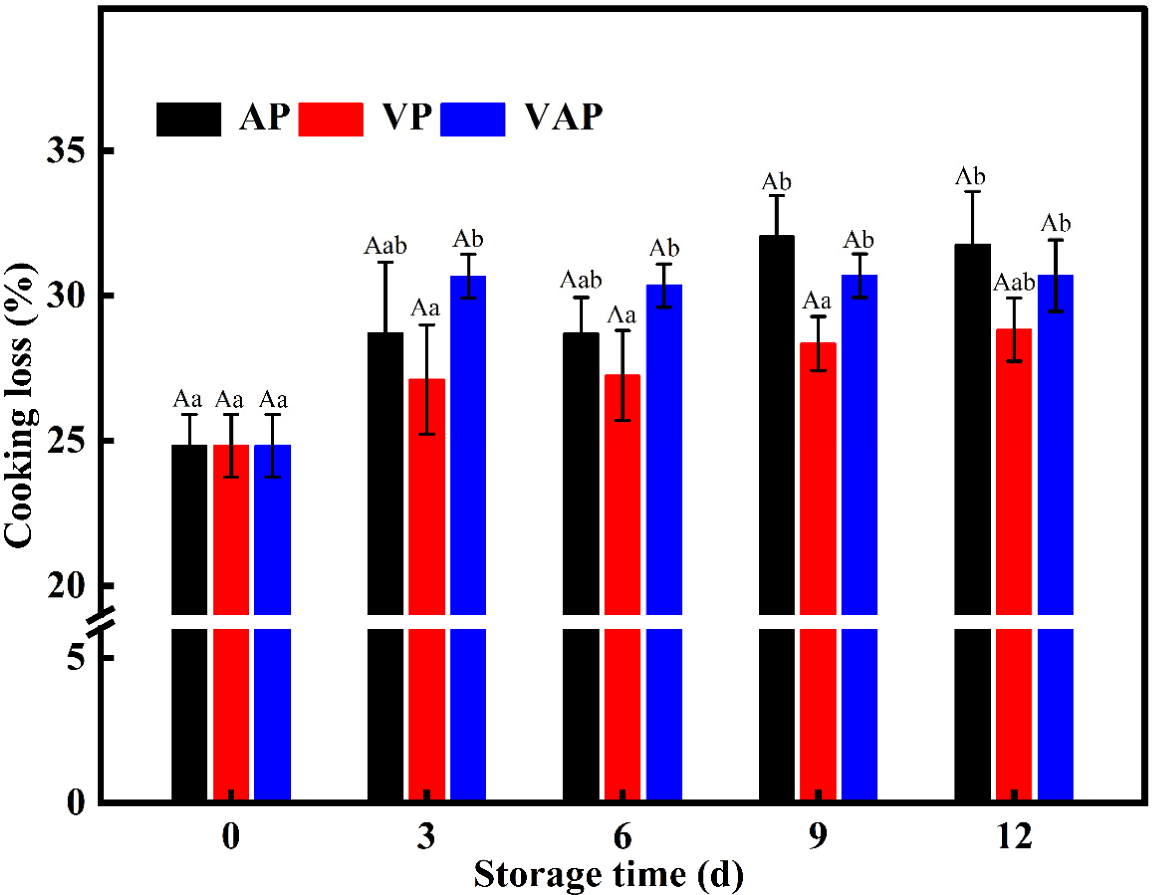
Cooking loss is an essential indicator for assessing meat quality, reflecting water loss from raw to cooked meat during processing. Fig. 2 showed no discernible difference in pork cooking loss rate between treatment groups over time (p>0.05). At 0–3 d of storage, the cooking loss increased significantly from 24.83% to 31.27% (p<0.05) in the VAP group, while it of AP and VP did not change significantly to 28.71% and 25.73%, respectively. From 3 to 12 d, the pork cooking loss in the AP and VP group did not change sharply with storage time. The increment of cooking loss in the VAP group continued to be significant and maintained between 30.34% and 31.59%. Most water in muscle is absorbed within the sarcoplasm of muscle fibers, with proteins in the plasma playing a major role in muscle water holding capacity (Honikel et al., 1986). Increased cooking loss during maturation may result from protein degradation at myofibrils, myoadipose fibers, and protein levels leading to myosin degeneration and weakened myofibrils, decreasing water holding capacity during storage.
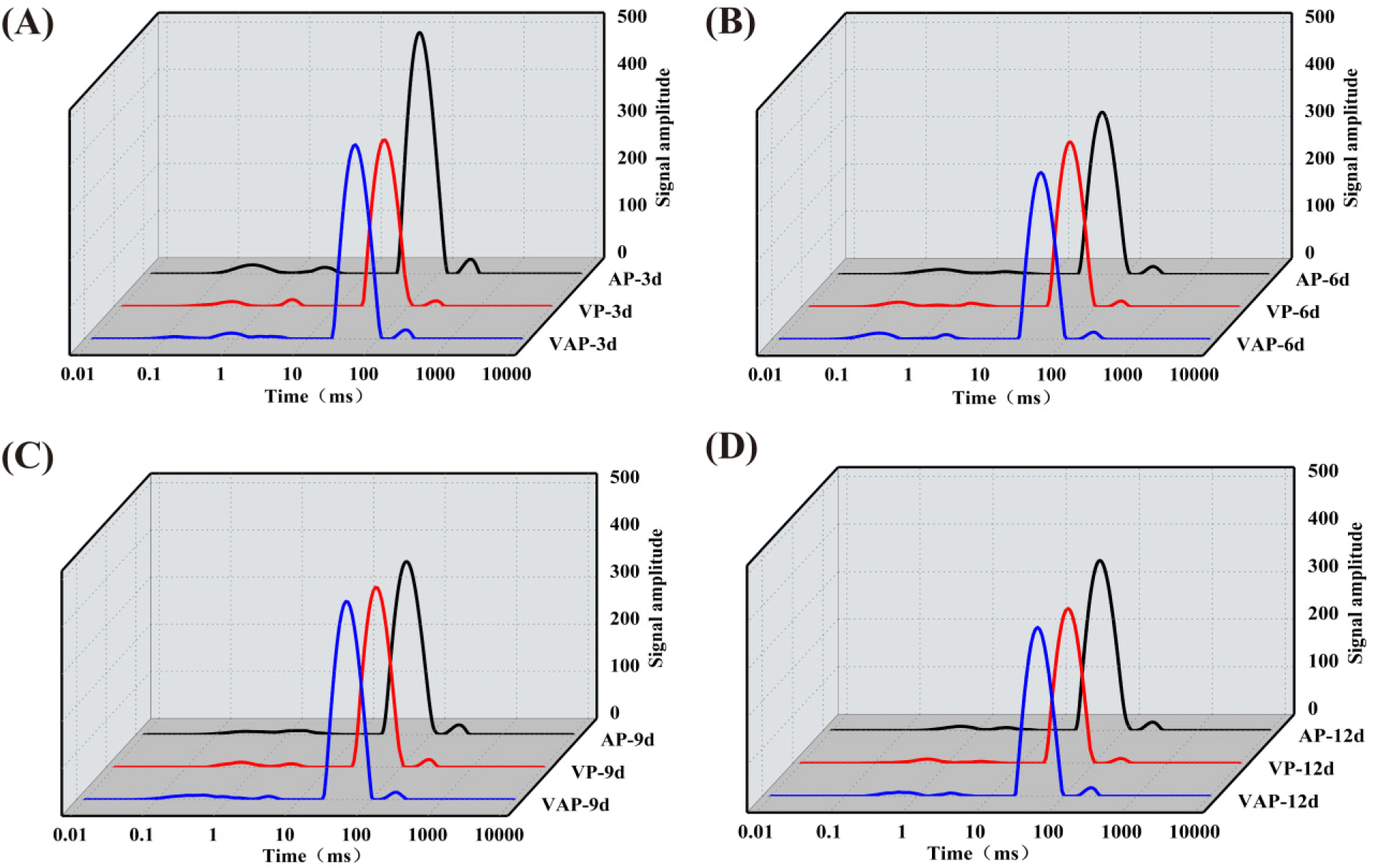
Low-field nuclear magnetic resonance is used to nondestructively detect water distribution and migration in samples. Changes in water composition and state in pork from different groups are displayed in Fig. 3. In meat, water exists in three states: bound water, immobilized water, and free water, with corresponding T2 relaxation time intervals of 0–10 ms (T2b), 10–100 ms (T21), and 100–1,000 ms (T22), respectively (Song et al., 2021). Similarly, P2b, P21, and P22 express the proportions of T2b, T21, and T22, respectively (Table 4). Immobilized water is the major state of water in raw muscle and cooked meat and is thought to be located between the thick and thin filaments of myofibrillar proteins (Honikel et al., 1986). After 3 d of storage, the content of immobilized water in the VP and VAP groups was obviously lower than that in the AP group. Li et al. (2022) pointed out that immobilized water is associated with myofibrillar structure and is easily lost due to myofibrillar protein degradation. Simultaneously, the TVB-N values of the samples in the corresponding phase showed the same trend and faster growth rate, which was consistent with the change of P21. At 6, 9, and 12 d of storage, the other two groups still showed lower levels compared with the AP group. According to Table 3, with the extension of storage time, the content of immobilized water in pork of all groups gradually decreased, and the content of free water increased. Structural damage to muscle tissue will gradually exude immobilized water from muscle fiber aggregates and convert it into free water (Xu et al., 2020). It was demonstrated that part of the immobilized water was converted to free water during beef ripening (Guo et al., 2023). During the entire storage, the highest free water content was observed in the AP group and the lowest in the VP group. A high proportion of free water indicates poor water holding capacity of the sample (Zhang et al., 2019). Consequently, the changes coincide with the cooking loss results. As the result shows, the main role of TiO2 in VAP group is focused on antibacterial and reducing the impact of microorganisms on protein degradation, and the impact on water molecule migration is relatively small compared with the VP group.
A,B At the same time point, different capital letters indicate a significant difference (p<0.05) between the treatments.
A physical characteristic called texture reflects the organization of meat tissue. Table 5 displays changes in textural parameters for pork in all treatment groups, including hardness, springiness, chewiness, cohesiveness, and gumminess. Consumers use tenderness as a key criterion to assess meat quality (Zhang et al., 2021). Meat stiffness, depending on connective tissue amount and quality, sarcomere length when muscle enters rigor, and proteolysis degree during cold storage, can indicate meat tenderness (Bao and Ertbjerg, 2019). At 0 d, the hardness, cohesiveness and adhesiveness of pork in all three treatment groups were at there maximum values during storage (Table 5). After 12 d of storage, the hardness, cohesiveness and adhesiveness of pork in the AP group exhibited significantly lowest values compared to those in other all groups (p<0.05), indicating that VP had a visible effect on maintaining pork texture at the end of storage. Moreover, the hardness, cohesiveness and adhesiveness of the pork in the VAP group were higher than those in the VP group, suggesting that the preservation effect of VAP was as expected. During storage, the hardness, cohesiveness and adhesiveness of pork in all three treatment groups exhibited a decreasing trend. TiO2 in VAP group has good antibacterial properties, which could reduce the fragmentation and looseness of muscle microfibers in fresh meat brought by microorganisms, thus exhibiting good (hardness, cohesiveness, and gumminess) performance in the VAP group as shown in Table 5. No significant differences in springiness and chewiness were observed, and the pattern of change was consistent with previous studies (Aguilera Barraza et al., 2015). This is in line with prior findings that as muscle fibers and proteins are broken down over time by microorganisms and enzymes, the flesh structure relaxes, resulting in decreased meat hardness in later stages of preservation (Li et al., 2019). Meat proteins lose distance and form new cross-bonds as a result of reduced moisture, leading to increased sample hardness (Bayram and Bozkurt, 2007). This is in accordance with the continuously growing trend of cooking loss rate. Degree of aggregation of myofibrillar proteins can lead to changes in the functional properties of muscle proteins, resulting in changes in texture (Li et al., 2019).
A,B At the same time point, different capital letters indicate a significant difference (p<0.05) between the treatments.
Conclusion
The VAP group exhibited superior preservation effect of pork and an extended shelf life of up to 12 d compared to the AP and VP groups. Furthermore, the VAP group displayed obviously lower levels of TVB-N and TVC values [14.70 mg/100 g, 5.85 lg (CFU/g)], maintained complete tissue integrity, and possessed a higher CIE a* at the end of storage. It can be concluded that antibacterial film incorporating TiO2 may inhibit microbial growth by generating reactive oxygen species, thereby slowing down the spoilage of chilled pork. In future research, the preservation effect of antibacterial composite film with TiO2 in commercial applications needs further investigation.













