Introduction
Camel milk serves as a vital nutritional resource for populations residing in arid and semi-arid environments, providing substantial nourishment along with notable health advantages and therapeutic properties (Ho et al., 2022). Compared to cow milk, camel milk boasts a higher fat content and protein concentration (Maryniak et al., 2018; Miao et al., 2023). Additionally, camel milk is abundant in unsaturated fatty acids and features lipid globules within a specific size range, enabling efficient absorption within the human body (Han et al., 2022). The fat and protein composition of camel milk closely resembles that of breast milk, suggesting its potential as a substitute for infants who are unable to consume breast milk (Xiao et al., 2023). Biologically active compounds found in camel milk, including α-lactalbumin, lactoferrin, immunoglobulins, and lactoperoxidase, contribute to enhanced intestinal enzyme digestion, decreased risk of allergic reactions, and notable antimicrobial properties against various pathogens (Alhaj et al., 2022; Behrouz et al., 2022). The immune-regulating functions of these molecules play a crucial role in protecting newborns from infections and promoting the growth and development of neonatal organs through the stimulation of specific gene expression patterns (Welle, 2023; Zhou and Pu, 2023). The biological functions of camel milk are largely dependent on the molecules present in its various components. Hence, the preservation of biologically active molecules in camel milk during processing is influenced by storage conditions. The stability of peptide chains is notably reduced in non-cryopreserved milk, with this decrease becoming more pronounced at elevated temperatures and extended storage durations (Howland et al., 2020). Additionally, there may be an increase in protease cleavage activity for arginine, lysine, and glutamate, impacting the biological functions of proteins and peptides (Howland et al., 2020). In contrast, the storage of fatty acids at 4°C for a duration of 96 hours is not found to have a significant impact on the levels of saturated, monounsaturated, and polyunsaturated fatty acids (Nessel et al., 2019). Nevertheless, it is important to note that during this timeframe, the concentrations of free fatty acids may experience a notable increase of 265%, potentially leading to heightened levels of oxidative stress in consumers (Nessel et al., 2019). Chronic consumption of lipid peroxidation products has been associated with growth retardation, intestinal irritation, cardiovascular disease, and carcinogenic effects. To optimize its efficacy, careful consideration should be given to the collection and storage methods of camel milk, while production processes should adhere to stringent hygiene and quality standards.
The limited supply of camel milk can be attributed to a number of factors, such as challenges associated with intensive camel farming and raw milk collection, the remote locations of breeding areas in relation to urban centers, and the restricted numbers of existing camel herds (Konuspayeva et al., 2023). The collection process for camel milk typically involves selecting a herd of camels, cleaning and disinfecting their udders, sterilizing collection equipment, regulating the timing and frequency of milk collection, and promptly cooling the milk after it has been collected (Paredes-Belmar et al., 2022). A segment of camel milk is gathered and preserved manually by herdsmen, without adherence to standardized production protocols (Konuspayeva et al., 2023). Consequently, the quality of raw camel milk may fluctuate due to inconsistent collection and storage practices. Furthermore, the maintenance of appropriate storage conditions is essential for controlling microbial levels in dairy products, including camel milk (Konuspayeva et al., 2023). The process of preserving camel milk generally consists of two separate phases: short-term preservation during transportation and long-term preservation at processing facilities (Konuspayeva et al., 2023). Nevertheless, there is a significant lack of contemporary studies addressing short-term preservation during transit. Improper handling and extended storage may lead to microbial contamination in raw camel milk, thereby jeopardizing its nutritional quality and undermining the purported health advantages associated with its consumption (Ali Redha et al., 2022). The predominant focus of global microbiological standards for dairy production is on cow’s milk, as noted by Konuspayeva et al. (2023). Therefore, it is crucial to conduct thorough research on the nutritional and microbial composition of camel milk, as well as establish baseline reference points for its transportation conditions. This information is vital for developing accurate collection and storage protocols for future camel milk products.
This research examined the influence of storage conditions on the Bactrian camel milk sourced commercially in Delingha City, Qinghai Province, China. Through a variety of experiments, such as sensory evaluation, colony counting, milk composition analysis, and 16s microbial detection, it was determined that long-distance transportation of camel milk using a 4°C milk truck is not suitable for preserving the quality and nutritional value of the product.
Materials and Methods
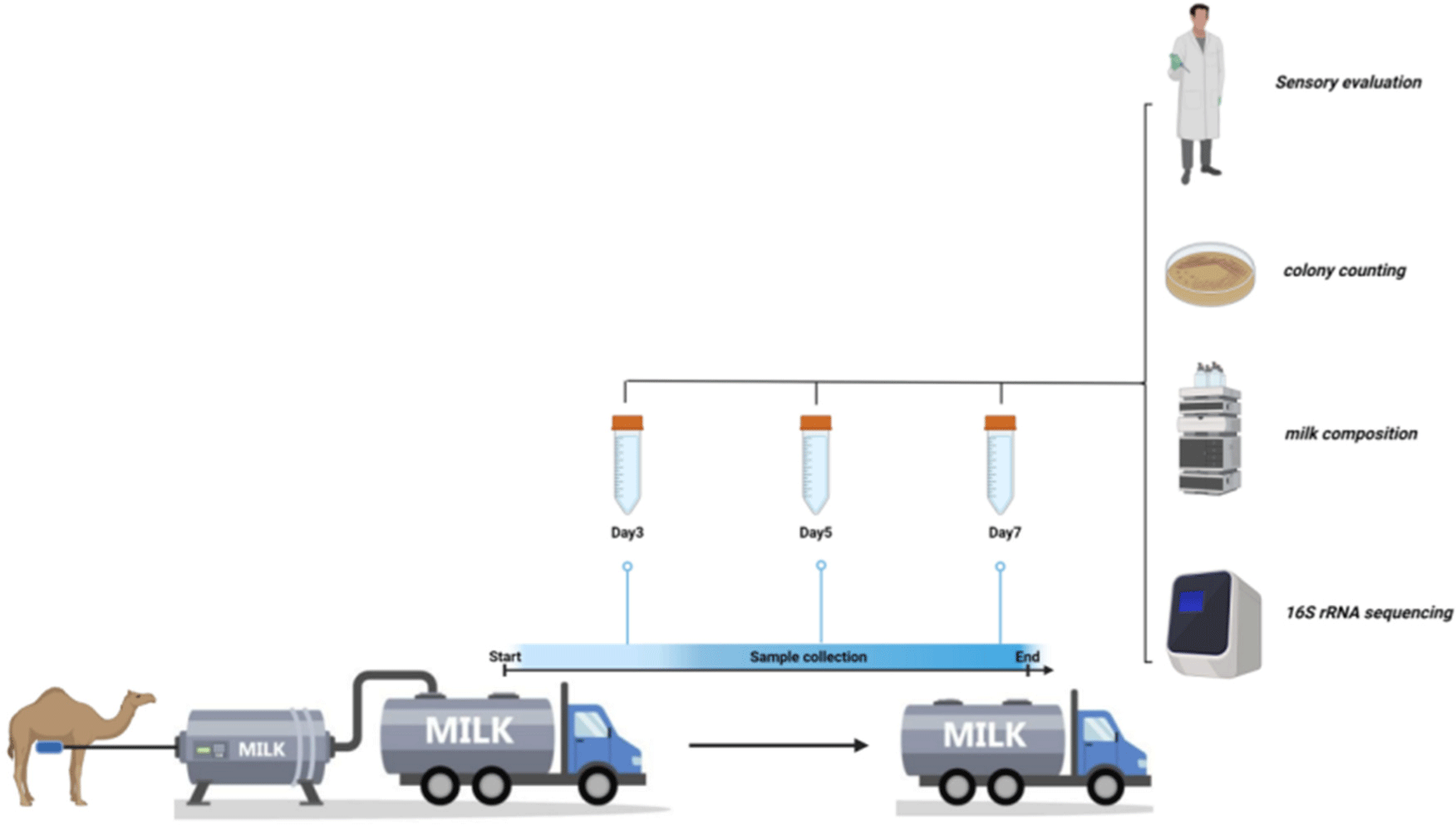
Thirty female camels were randomly selected from the ranch pasture for milking after disinfection by the same herder. The raw milk was then placed in cleaned milk storage containers and cooled in a refrigerator at 4°C. Subsequently, the cooled raw milk was rapidly cooled to approximately 4°C and transferred to a refrigerated milk truck’s cleaned storage tank via insulated pipelines. The temperature of the milk storage tank in the refrigerated milk truck was carefully maintained at 4°C during transportation. Milk samples should be collected on the 3rd, 5th, and 7th days and stored in 50 mL sterile, enzyme-free cryotubes. Subsequently, the samples should be transported to the laboratory for further analysis using liquid nitrogen transportation. The specific process is shown in Fig. 1.
The candidates for the evaluation team were chosen from the student body of Qinghai University and underwent two rounds of assessment. The initial round focused on discerning odors and tastes, with successful participants advancing to a subsequent round involving taste tests of pasteurella and skimmed camel milk. Those students who demonstrated proficiency in identifying samples were ultimately selected to serve on the evaluation panel. Obtain a suitable sample size and transfer it to a 50 mL beaker for observation under natural light. Assess the color and tissue integrity, smell the odor, and rinse the mouth with warm water to evaluate the flavor. The color should appear as milky white or slightly yellow, while the taste and odor should exhibit a characteristic milk aroma without any off-putting smells. The consistency of the sample should be uniform and liquid, free of clots, precipitates, or visible foreign matter when examined with normal vision. After completion of the assessment, the evaluation team members described the samples evaluated.
The enumeration of bacterial colonies in milk samples was conducted utilizing the standard plate count technique. Prior to the commencement of the study, essential culture media, plates, and equipment were sterilized via autoclaving. Furthermore, the experimental workspace and environment were subjected to ultraviolet light exposure for a duration exceeding 30 minutes to mitigate potential environmental contaminants during the experimental procedures. Each milk sample was thoroughly mixed, and 1 mL of camel milk sample was taken for bacterial counting. After consecutive 10-fold dilutions with 0.9% sodium chloride solution, 1 mL of each dilution was transferred to duplicate culture dishes. Approximately 15–20 mL of Potato Dextrose Agar was melted and poured into the culture plates, allowed to solidify, and then incubated at 28°C for 5 days for mold and yeast counting (Makki et al., 2020). Similarly, 15–20 mL of violet red bile agar (VRBA) was melted and poured into culture plates, mixed well with the sample, solidified, and then overlaid with 3–4 mL of VRBA for cultivating Escherichia coli, incubated at 37°C for 24 hours for counting (Kadri et al., 2021). Finally, 12–15 mL of Nutrient Agar was solidified and incubated aerobically at 37°C for 48 hours for total colony counting (expressed as colony forming units per milliliter, CFU/mL; Huang et al., 2019).
A milk sample weighing 9 grams was placed in a 100 mL conical flask, to which 3 drops of 1% phenolphthalein solution were added. The acidity of the sample was subsequently assessed via titration with 0.1 mol/L NaOH (Oselu et al., 2022). The pH of the sample was determined utilizing a pH meter. The milk samples were dried in an oven (OMH180-S, Thermo Fisher Scientific, Waltham, MA, USA) at 105°C until a constant weight was achieved. The fat content was determined using Gerber’s method (He et al., 2019), and subtracting this value from the total solids content provided the non-fat milk solids content (Alfaris et al., 2022). The lactose content was determined using high-performance liquid chromatography (McCune et al., 2023), while the protein content was determined through the Kjeldahl method (FOSS 8420), with the total protein nitrogen content being calculated at 6.38 times (Tanaka et al., 2023). Finally, the amino acid content of the samples was determined via an automatic amino acid analyzer (S433D, Sykam, Eresing, Germany; Salmen et al., 2012).
Camel milk samples were transported on dry ice to the detection center of Shenzhen Huada Gene Technology Services (Shenzhen, China) for 16S rRNA amplicon sequencing. DNA extraction from the camel milk involved centrifuging the samples at 10,000×g at 4°C for 10 min to remove the fat layer and supernatant. The DNA from the precipitate was then extracted using MagPure Stool DNA KF Kit B. This extracted DNA underwent library preparation and amplicon sequencing, in which primers for the bacterial V4 region (515F: GTGCCAGCMGCCGCGGTAA, 806R: GGACTACHVGGGTWTCTAAT) were used to amplify the variable region of the bacterial 16s rRNA gene. The amplified 16s rRNA gene was generated by PCR (S100, Bio-Rad Laboratories, Hercules, CA, USA) under specific conditions. Libraries were constructed by purifying and dissolving the samples in Elution Buffer using Agencourt AMPure XP magnetic beads (Beckman, Brea, CA, USA). The fragment range and concentration of the library were analyzed using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA), and the qualified samples were subsequently sequenced by Illumina (San Diego, CA, USA; MiSeq) after passing quality control measures.
The hypervariable region tags were obtained by stitching together reads larger than 15 bp with a mismatch rate of overlapping regions less than 0.1 from the raw sequencing data, resulting in continuous sequences. These tags were then clustered based on a 97% sequence similarity threshold to generate operational taxonomic units (OTUs). The obtained OTUs were compared against the v20110519 database to remove PCR-generated chimeras. All tags were aligned with the representative sequences of the OTUs using the usearch_global method, and the abundance of each OTU was determined for each sample. The representative sequences of the OTUs were aligned with the Greengene v201305 database for species annotation, using a confidence threshold of 0.6, OTUs without species annotation and OTUs assigned to non-bacterial species were removed before proceeding with data analysis.
The data were subjected to averaging with SD, utilizing the ANOVA method in SAS software (version 8.0, SAS Institute, Cary, NC, USA) for analysis of variance. In cases of significant differences, Duncan’s multiple range test will be employed for comparative analysis. It was assumed that there was no statistical difference between the 5-day and 7-day groups compared to the 3-day group. The analysis focused on the differences between the 5-day and 7-day groups compared to the 3-day group, with statistical differences recognized when p<0.05.
Results
The milk sample can be stored in a refrigerated truck at 4°C for 3 to 5 days, during which time the taste and texture of the product remain satisfactory. However, after 7 days of storage, there was an increase in the amount of fat floating and a uniform layer of fat formed on the surface of the sample liquid, as indicated in Table 1.
Based on the total bacterial count test results, the microbial findings of the milk samples stored in the milk tanker for 3 and 5 days fell within the established control parameters, whereas a substantial increase in colony formation was observed after 7 days (Table 2).
| Times (d) | Mold (CFU/mL) | Yeast (CFU/mL) | Escherichia coli (CFU/mL) | Total colony count |
|---|---|---|---|---|
| 3 | 0 | 3 | 1 | 9 |
| 5 | 0 | 7 | 4 | 13 |
| 7 | 4 | 8 | 7 | 35 |
Table 3 displays protein analysis of camel milk samples from various days, showing a decrease in protein content in 5-day and 7-day samples compared to the 3-day sample. Table 4 reveals amino acid analysis, with non-essential amino acids surpassing essential ones. Glutamic acid was the most abundant amino acid. Prolonged storage resulted in notable reductions in arginine, leucine, methionine, and isoleucine in camel milk.
Saturated fatty acids were found in higher levels (70.6%–80.4%) than unsaturated fatty acids (19.6%–29.4%), with low amounts of short-chain and medium-chain fatty acids. The most abundant fatty acids were C16:0 (33.3%–38.6%), C18:0 (17.2%–26.5%), and C14:0 (14.7%–16.4%). C18:1n-9 had the highest content among unsaturated fatty acids, ranging from 1.9%–22.1%. Conjugated linoleic acid content was found to be 84.8–372 mg/kg, as shown in Table 5.
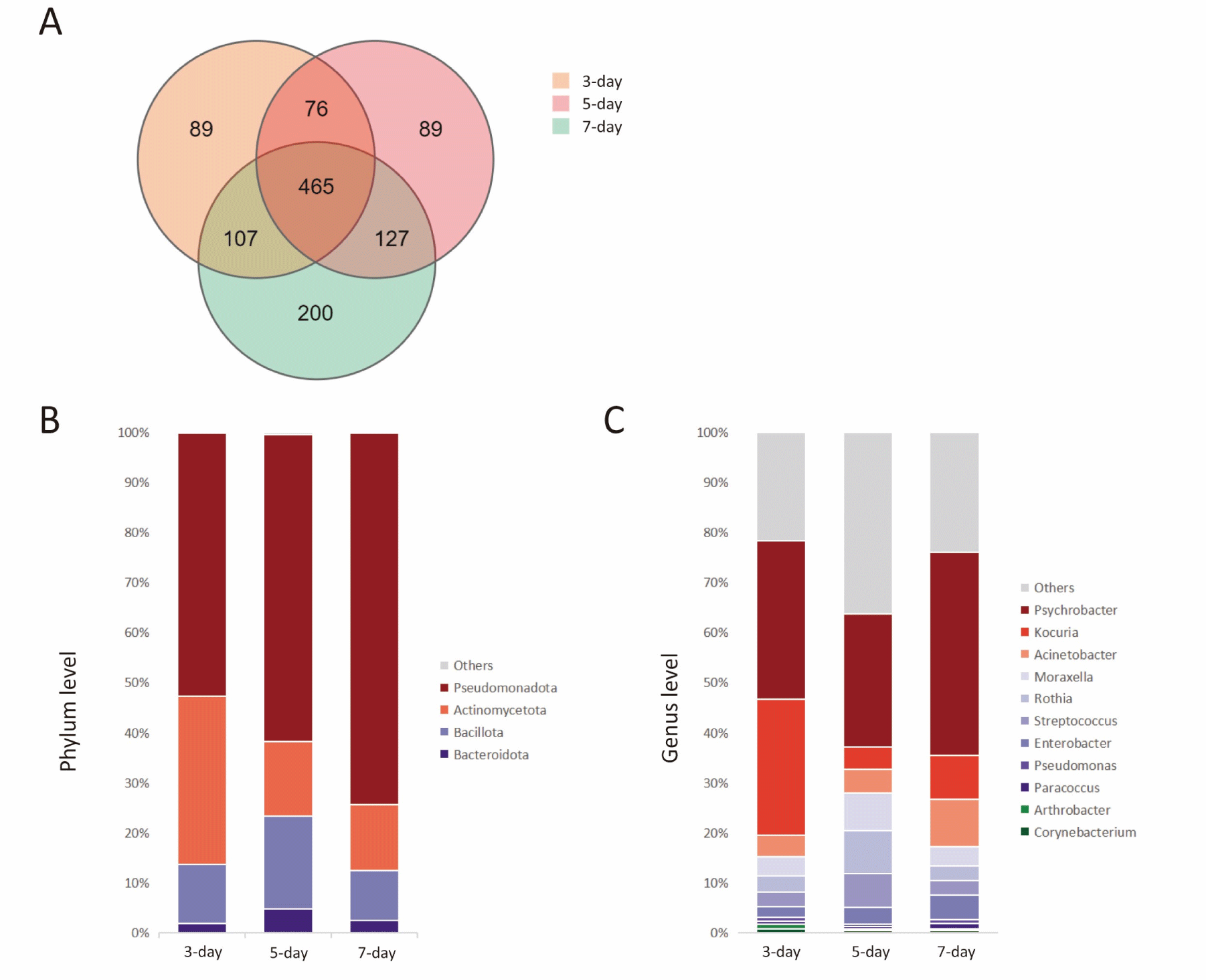
Three samples produced 231,818 paired reads, resulting in 230,815 clean reads and the identification of 2,392 OTUs. Pseudomonadota was the dominant phylum at 61.9%, followed by Actinobacteria (20.4%), Bacillota (13.2%), and Bacteroidota (3.0%). The most abundant genera were Psychrobacter (32.9%) and Kocuria (13.5%), with other genera over 1% including Acinetobacter (6.2%), Moraxella (5.1%), Rothia (4.9%), Streptococcus (4.2%), and Enterobacter (3.5%). The microbial composition of camel milk samples changed over time during storage, with an increase in Pseudomonadota abundance as storage duration increased. Conversely, the abundance of Actinomycetota decreased over time, representing 33.5% in the 3-day group, 14.7% in the 5-day group, and 13.1% in the 7-day group (Fig. 2B). Moreover, the abundance of Psychrobacter at the genus level, a member of the Pseudomonadota phylum, exhibited a notable increase over the observation period, comprising 31.6% in the 3-day group and 40.5% in the 7-day group. In contrast, the abundance of Kocuria, a member of the Actinomycetota phylum, demonstrated a decrease over time, mirroring the trend observed for Actinomycetota, with proportions of 27.2% in the 3-day group and 8.7% in the 7-day group (Fig. 2C).
Discussion
This study investigated how storage conditions affect commercially sourced Bactrian camel milk in Delingha City, Qinghai Province, China. Results showed that transporting camel milk long distances in a 4°C milk truck is not ideal for maintaining quality and nutrition. The composition of camel milk exhibited stability upon initial storage, however, prolonged storage resulted in notable alterations in protein and fat content. This phenomenon may be attributed to the metabolic activity of specific bacteria such as Psychrobacter and Kocuria, which metabolize fat and protein for energy in lieu of lactose.
Camel milk has lower levels of casein and higher levels of functional whey proteins than cow’s milk (Miao et al., 2023). The decline in total protein content in camel milk results in a reduction of functional proteins, which decreases its antioxidant, antidiabetic, antihypertensive, antibacterial, and anticancer attributes. The amino acid composition in the 3-day group was consistent with previous studies, but levels of arginine, methionine, isoleucine, and leucine decreased over time in the 5-day and 7-day groups, possibly because of the increased cleavage activity of proteases for amino acids such as arginine, which affects the biological function of proteins and peptides (He et al., 2019; Howland et al., 2020; Miao et al., 2023). Low arginine levels in camel milk support growth in young individuals, but prolonged storage can affect its functionality (Kim et al., 2004; Zhao et al., 2015). Protein oxidation during storage reduces methionine content, impacting milk stability and nutritional value (Baxter et al., 2007). Decreased leucine and isoleucine in whey protein suggest lower protein levels in camel milk, impacting its functionality (Da Silva et al., 2017; Rafiq et al., 2016; Roucher et al., 2013). Camel milk exhibits a higher concentration of unsaturated fatty acids, specifically linoleic acid and eicosapentaenoic acid, compared to cow’s milk, thereby rendering it a healthier alternative (Miao et al., 2023). Camel milk samples showed a decrease in unsaturated fatty acids, possibly influenced by various factors like geography, diet on camels (He et al., 2019; Morales-Almaráz et al., 2011). On the other hand, it may also be affected by preservation conditions and microbial activity leading to nutrient degradation (Narmuratova et al., 2006; Nessel et al., 2019). Previous research focused on unsaturated fatty acids in milk stored at 4°C for up to 96 hours, but this study examined the effects of real-life storage conditions on camel milk, leading to lipid peroxidation and the formation of lipid peroxides (Nessel et al., 2019). Furthermore, recent studies have indicated that the antioxidant properties of vitamin C in camel milk may degrade after 24 hours at 4°C, potentially exacerbating lipid peroxidation in the polyunsaturated fatty acids present (Nessel et al., 2019). Additionally, a decrease in short-chain fatty acids and medium-chain triglycerides was observed in comparison to earlier research, suggesting that microorganisms may be utilizing these compounds as a source of energy (Chilliard et al., 2000).
The analysis revealed that Pseudomonadota, Actinomycetota, Bacillota, and Bacteroidota were the predominant phyla in the samples, exhibiting greater variability in comparison to previous studies conducted in Kuwait, Inner Mongolia, and Morocco (Kadri et al., 2021; Rahmeh et al., 2022; Zhao et al., 2020). Particularly noteworthy was the increased abundance of Pseudomonadota and Actinomycetota in this study, with a wide range of genera present within these phyla (Kadri et al., 2021; Rahmeh et al., 2022; Zhao et al., 2020). Psychrobacter and Kocuria were identified as prevalent genera at the genus level. Psychrobacter, belonging to the phylum Pseudomonadota, is commonly found in raw milk and agricultural settings, demonstrating an ability to thrive in cold storage conditions and consume fat present in raw milk (Bowman, 2006; Welter et al., 2021). Kocuria, a member of the Actinomycetota phylum, is known to produce enzymes essential for cheese ripening; however, its enzymatic activity diminishes with extended storage at low temperatures (Beresford and Williams, 2004). In contrast, the levels of Acinetobacter, Moraxella, Rothia, Streptococcus, and Enterobacter in raw camel milk remain relatively stable during storage (Kadri et al., 2021; Rahmeh et al., 2022; Zhao et al., 2020).
Alterations in the microbiota of camel milk can have implications for food safety. Pasteurization is essential when utilizing camel milk as a health product to eradicate the majority of Psychrobacter species and mitigate the likelihood of bacterial infections; however, it may not completely eradicate infections caused by thermophilic bacteria (Masotti et al., 2023). Of the five Kocuria genera that displayed significant prevalence in the research, certain strains are recognized as opportunistic pathogens, presenting a heightened risk to individuals with compromised immune systems and potentially resulting in infectious conditions such as bacteremia and peritonitis (Ziogou et al., 2023). The identification of the genus Acinetobacter in contaminated dairy products, although infrequently associated with diarrheal illness, is worrisome because certain strains demonstrate resistance to multiple drugs, thereby increasing the likelihood of infection in vulnerable populations such as immunocompromised individuals and young children (Wisplinghoff et al., 2012). While the levels of Moraxella, Rothia, Streptococcus, and Enterobacter were not significantly impacted by storage duration, these bacteria also pose inherent risks of infection (Davin-Regli et al., 2019; Maraki and Papadakis, 2015; Zbinden et al., 2015). Traditional pasteurization can kill harmful bacteria in camel milk but may also change its nutritional value. New preservation techniques for camel milk are needed to address this issue.
Several methodologies have been explored in prior research endeavors to mitigate microbial contamination. Initially, a comprehensive cleansing of the storage tank prior to each loading operation serves to eradicate biofilms and diminish bacterial presence (Darchuk et al., 2015). Additionally, prompt cooling of milk to 4°C within a two-hour window following collection aids in the retention of essential nutrients (Ajmal et al., 2018). While the conventional practice involves storing raw milk at 4°C until transportation, certain scholars have investigated supplementary interventions. Low CO2 pressure slows bacterial growth without protein precipitation (Rajagopal et al., 2005). Rinsing raw milk with N2 gas inhibits bacterial growth, even against multi-drug resistant strains (Munsch-Alatossava and Alatossava, 2020). Cryopreserving raw camel milk reduces microbial reproduction but results in loss of lactoferrin and lysozyme (Leclair et al., 2019; Paulaviciene et al., 2020). These methods are not practical for small farms due to costly equipment and gas cylinders. Ultrasonic techniques have demonstrated potential as a viable alternative to pasteurization, effectively inhibiting microbial growth while preserving biological activity when applied at power levels ranging from 105 to 140 W (Dhahir et al., 2020; Mudgil et al., 2022). Employing ultrasonic treatment within this power range for the preservation of raw camel milk represents a promising strategy for extending its shelf life.