Introduction
Dairy farming is vital to the agricultural industry as a supplier of essential products such as milk and cheese. However, recent environmental changes have also affected cattle health and milk production (Bokharaeian et al., 2023; Guzmán-Luna et al., 2022). Therefore, ensuring the quality and safety of dairy products is of paramount importance to consumer health. It is also important for animal welfare and behavior (Castellini et al., 2023).
Stress has been identified as a factor that can negatively affect the health and welfare of animals, leading to lower milk production and quality (Bokharaeian et al., 2023; Park et al., 2022). Heat stress can alter the biological composition of cattle by affecting hormone secretion, immune responses, and milk composition (Ma et al., 2019). In animal studies, cortisol levels are commonly monitored to detect stress responses and potential welfare issues (Ito et al., 2017). Cortisol can be detected in common biomatrices, such as blood, saliva, urine, hair, and milk, involving both stressful and non-stressful procedures to evaluate whether an animal’s condition is normal or abnormal (Ataallahi et al., 2022; Ghassemi Nejad et al., 2022). Changes in cortisol levels in the bloodstream can affect milk cortisol concentration (MCC). The measurement of MCC is useful because abnormal levels have been linked to cattle stress or health conditions during milk production (Ataallahi et al., 2023; Fazio et al., 2015; Sgorlon et al., 2015).
Heat treatment is the most common processing method used to inactivate harmful components of dairy milk to ensure food safety and extend the shelf life of dairy milk products (Čurlej et al., 2022; Franzoi et al., 2022). Heat processing changes the structure of milk components such as proteins (Genene et al., 2019; Moro et al., 2019), vitamins, and volatile flavors (Coutinho et al., 2018) depending on the heat exposure of the milk during processing (Kilic-Akyilmaz et al., 2022; Lykholat et al., 2016). Monitoring the MCC in commercially processed milk products may be an alternative and noninvasive method for assessing the health and welfare of dairy cattle at the time of milk production. In addition, it may help ensure the safety and quality of processed dairy products. Therefore, this study aimed to explore the effects of various thermal processing methods, including low temperature, long duration, and high temperature, short duration on the cortisol concentrations in milk obtained from farms and markets.
Materials and Methods
Three healthy Holstein dairy cattle (30 to 70 months old) with a milk yield between 19 and 24 liters, and a milk composition of 4.0% fat and 2.8% protein were used in the current study. They were housed in open pen with a winch curtain. The dairy cattle were fed with concentrate (12 kg/head/day), ray grasses (Lolium) and Sudan grasses (Sorghum × drummondii). All cattle were administered water ad-libitum. They belonged to a livestock research farm located at Kangwon National University in Chuncheon, Korea.
Farm milk samples (n=36) were obtained within two days. Each sample was placed in a conical tube (50 mL) during regular milking in the morning (08:00) and afternoon (16:00). The sample tubes were placed in a cooled transport bag and transported to the laboratory and refrigerated at 4°C.
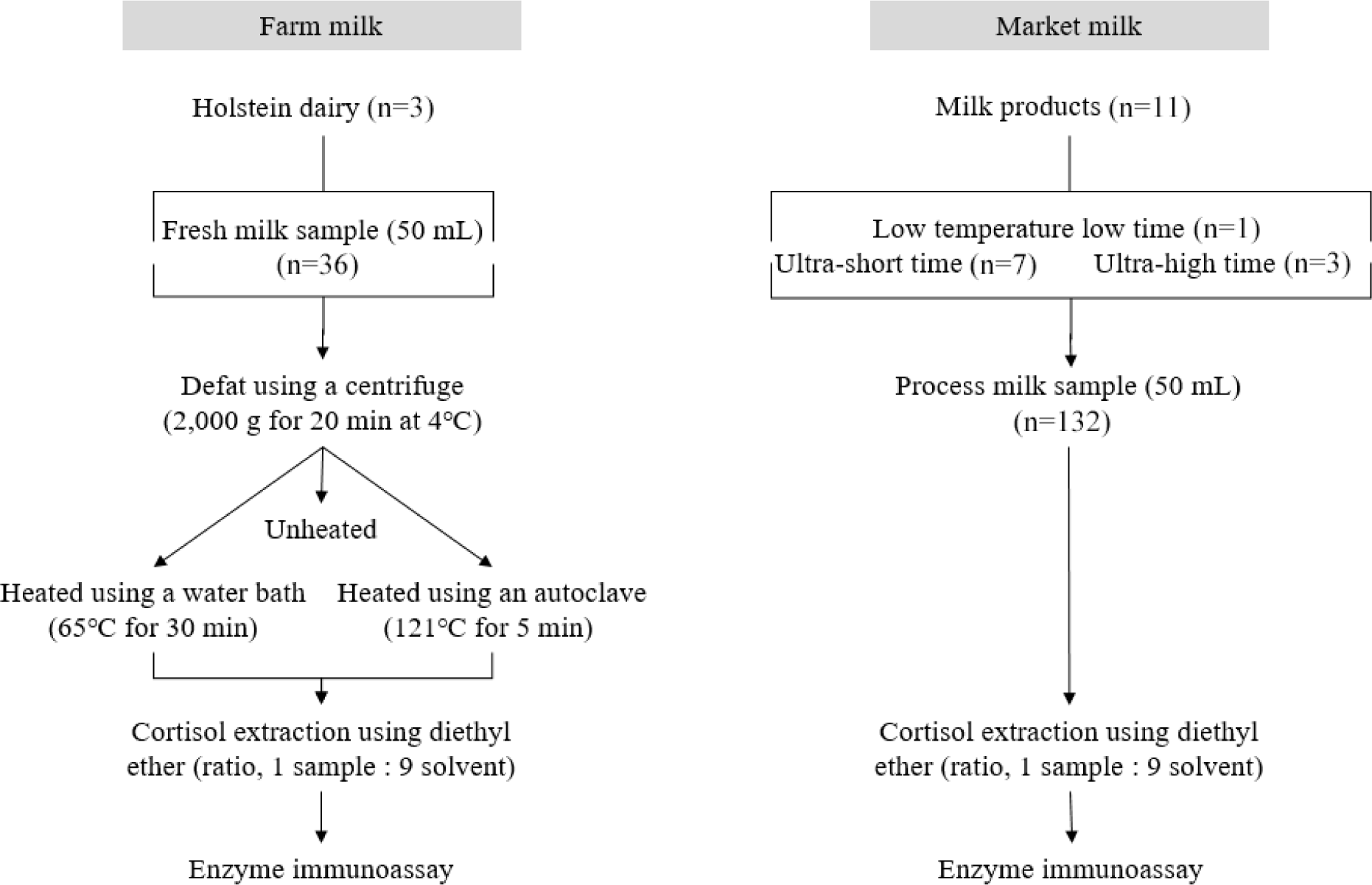
The whole milk tubes were vortexed, then centrifuged at 2,000×g for 20 min at 4°C and milk fat was removed from the surface (Fukasawa et al., 2008). Then, the skim milk was divided into three portions in new conical tubes. One part as a raw skim milk (unheated), the other two parts of skim milk were heated at 65°C for 30 min using a laboratory water bath (Wesolowska et al., 2019), and heated at 121°C for 5 min using an autoclave (Papapanagiotou et al., 2005). Milk samples were thoroughly mixed. The first aliquot of skim milk sample was placed in a water bath heated to 65°C for 30 min. The samples were then left to cool inside the water bath to 4°C. The same procedure was used for the second aliquot with the autoclave heated to 121°C at 5 min (Fig. 1).
Eleven commercial dairy milk products were purchased for analysis. All market milk products were made from 100% raw milk and they were heated by either low temperature low time (LTLT) at 63°C for 30 min, ultra-short time (UST) at range 120°C to 130°C for 2 to 3 seconds, or ultra-high temperature (UHT) at range 135°C to 150°C for 1 to 4 seconds. For each milk product, 50 mL was sampled in a conical tube for subsequent analysis. No additional preparation steps such as centrifugation were required (Fig. 1).
MCC was analyzed using the procedures described by Ataallahi et al. (2023) and Fukasawa et al. (2008). For cortisol extraction, the milk samples were thawed in a 37°C water bath and 0.1 mL of milk was mixed with 0.9 mL diethyl ether under a fume hood. The mixture was vortexed for 1 min and two organic (ether phase) and inorganic (aqueous phase) layers were formed. The organic layer containing cortisol was transferred into a separate 2 mL microcentrifuge tube and evaporated under a laboratory fume hood for 2 h. The extracted residues were stored at –20°C for further analysis. It should be noted that multiple extractions (two or three times) were sufficient for the full extraction of cortisol, and the amount of evaporated organic layers should be measured for the final calculation. The dried evaporated residue was re-dissolved in 0.25 mL of assay buffer supplied with a colorimetric competitive enzyme immunoassay (ELISA) kit (Enzo Life Science, Farmingdale, NY, USA) at room temperature, vortexed for 1 min and 0.1 mL of the reconstituted sample was assayed in duplicate in a 96-well plate (Fig. 1). The MCC of morning and afternoon milk samples were measured individually. The sensitivity of the cortisol ELISA kit was 56.72 pg/mL. The OD of the samples was measured at a wavelength of 405 nm using a microplate reader (SpectraMax Absorbance Reader, Molecular Devices, San Jose, CA, USA). MCC was reported in ng/mL. The average coefficient of variation (CV%) calculated for farm MCC was 9.2% for the intra-assay and 14.4% for the inter-assay. The average CV% calculated for the commercial MCC was 15.4% for the intra-assay and 19.8% for the inter-assay.
Statistical analysis was performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). To compare the farm MCC before and after heat processing, as well as the MCC in commercial milk products, an analysis of variance (ANOVA) was used. Data were considered statistically significant at p<0.05. All results are reported as mean±SD.
Results and Discussion
The results revealed that the average MCC (ng/mL) in unheated milk, heated milk at 65°C, and heated milk at 121°C, were 0.88±0.16, 0.86±0.19, and 0.80±0.15 respectively (Fig. 2). Accordingly, there was no significant difference (p>0.05) in MCC between unheated and heat-treated milk samples or between the heat treatment methods.
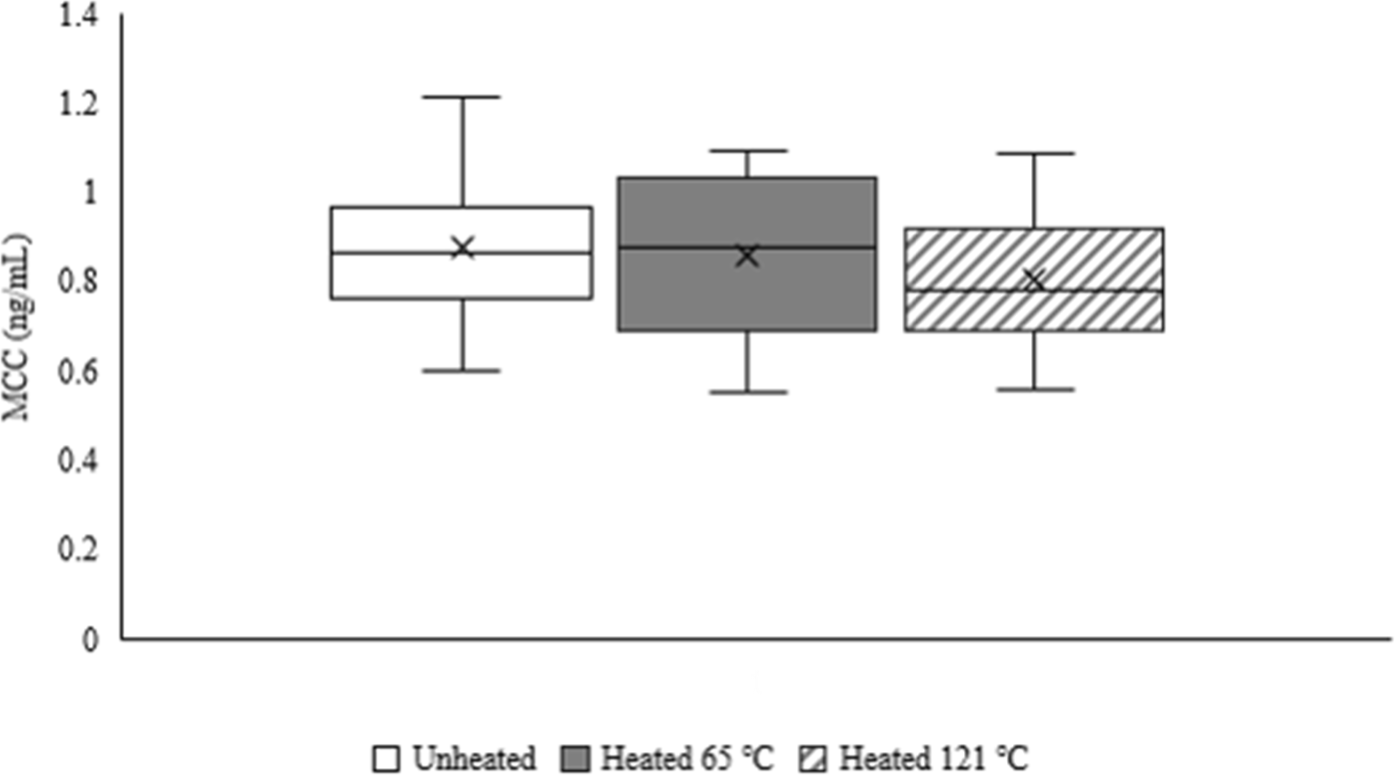
Cortisol, the main stress hormone in animals, is found at high levels in the blood and can be transferred to the mammary glands of dairy animals (Hechler et al., 2018). The range of MCC from 0.5 to 11.7 ng/mL was observed in previous studies (Gellrich et al., 2015; Verkerk et al., 1998). In addition, Malekinejad and Rezabakhsh (2015), reported the range of glucocorticoid hormone concentrations to be between 0.46 to 18 ng/mL in dairy milk, 20 to 136 ng/mL in human milk, and 144 ng/mL in rat milk. In our study, the average farm MCC was 0.83±0.17 ng/mL, falling within the range of glucocorticoid concentrations (0.7 to 1.4 ng/mL) previously reported by Jouan et al. (2006) in milk products. We observed no significant differences in the MCC before and after heat processing. This is consistent with previous studies indicating that steroid hormones are unaffected by heat because cholesterol, a key substrate for steroid biosynthesis, comprises stable carbon-based ring structures, making the molecule more stable below its melting point of 148.5°C (Derewiaka and Molińska, 2015; Malekinejad and Rezabakhsh, 2015; Van Der Voorn et al., 2017). In the dairy products industry, thermal processing is typically performed at temperatures ranging from 63°C to 100°C (Franzoi et al., 2022; Snoj et al., 2018). We found that that temperatures between 65°C for 30 min and 121°C for 5 min, unaffected the level of cortisol.
The results revealed that the average MCC (ng/mL) of commercially milk products processed under LTLT, UST, and UHT were 0.16±0.07, 0.15±0.08, and 0.15±0.07, respectively (Fig. 3). No significant differences (p>0.05) were observed in the MCC between commercial milk products subjected to different heat processing methods.
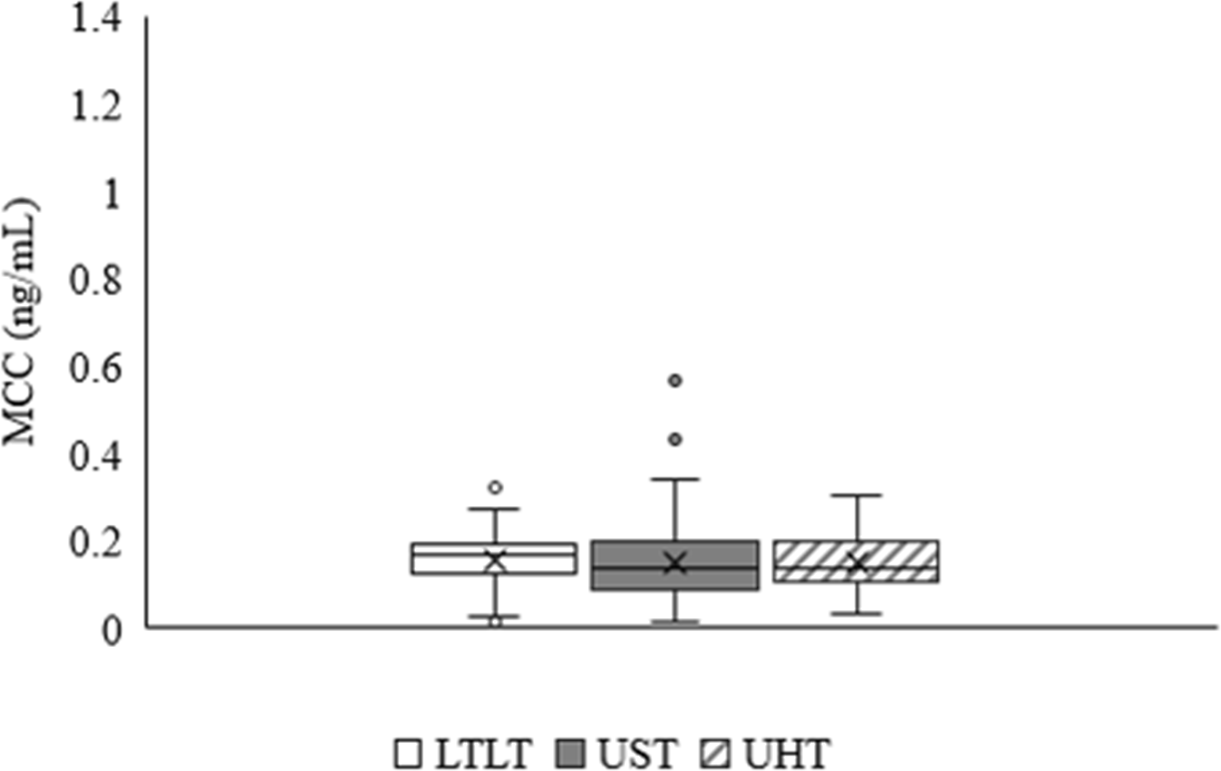
Heat treatment is known to influence the composition of milk depending on the intensity, duration, and temperature of the storage conditions (Krishna et al., 2021). Previous studies have indicated that protein-based hormones, including insulin-like growth factors, growth hormones, leptin, adiponectin, and insulin, are more susceptible to denaturation during heating than lipid-based hormones such as cortisol (Arslanoglu et al., 2013; Van Der Voorn et al., 2017; van Lieshout et al., 2020). In this study, despite employing different heat treatments (LTLT, UST, and UHT), no significant differences were observed in the MCC, suggesting that the heat processing methods did not significantly affect the MCC. This finding suggests that cortisol residue in milk remains relatively stable despite variations in the heat treatment processes commonly used in commercial dairy products (Ataallahi et al., 2023; Malekinejad and Rezabakhsh, 2015). Cortisol is a 21 carbon steroid with the characteristic 3-keto-4-ene structure of active steroids (Honour, 2022). The structural stability of cortisol is because of presence of strong covalent bounds within the molecule, particularly the bounds between carbon atoms in the rings and the hydrogen atoms attached to them. The composition of milk is a complex mixture of proteins, fats, carbohydrates, and other ingredients. The stability of cortisol in milk can be affected by interactions with these components. Cortisol may form complexes with proteins present in milk, which can affect its degradation by enzymes.
Under normal conditions, 80% to 90% of circulating cortisol is bound to corticosteroid- binding globulin and 10% to 15%, is bound to albumin, and remaining 5% to 10% cerculate as free and active hormone (Mormède et al., 2007). The basic molecular structure of cortisol remains the same whether its in the bloodstream or present in milk. But, when cortisol is found in milk, it may be in lower levels compared to its levels in the bloodstream, and it might also be found to carrier proteins specific to milk. Moreover, cortisol in milk might undergo some metabolic processes or interactions with other milk components, although these alterations typically do not change the fundamental structure of cortisol molecule itself. The exact extent of structural changes to cortisol during heat treatment can vary depending on factors such as temperature degree, duration of heating, and presence of other milk ingredients. High temperatures can lead to the denaturation of proteins, and it may affect cortisol indirectly by these processes.
Therefore, the stress levels in farm animals can be a determining factor affecting milk quality. The presence of cortisol in milk, a steroid hormone produced by the adrenal glands, has been linked to environmental stressors and poor welfare conditions in cattle (Jouan et al., 2006; Qu et al., 2018). However, only a small amount of glucocorticoid is transferred from the bloodstream to milk (Jouan et al., 2006; Qu et al., 2018). Poor welfare conditions not only affect milk production, leading to lower fat and protein levels (Castellini et al., 2023; Kawonga et al., 2012) but also affect the hormonal composition of milk. Despite the potential influence of poor welfare conditions on milk composition, our results suggest that the heat treatments commonly employed in the dairy industry do not significantly alter cortisol levels.
It is essential to note that milk quality is affected by various factors such as thermal processing (pasteurization and sterilization). Although effective at eliminating harmful components and bacteria, these processes may degrade heat-sensitive vitamins and enzymes, thereby reducing the nutritional value of milk (Macdonald et al., 2011; Vass et al., 2020). Furthermore, they may not effectively eliminate hormonal residues such as cortisol and estrogen. Understanding the stability of MCC under different heat treatment conditions has important implications for the dairy industry and product quality. This information will help improve the safety and quality of dairy products, while considering the welfare of dairy cattle. In addition, it will be explained whether the level of cortisol in milk and even processed milk can be used as an indicator of the overall good welfare of dairy farms. Monitoring hormonal residues, such as cortisol, in commercial processed milk may be a good parameter to evaluate the quality of milk and dairy products in the market.
Conclusion
Cortisol in farm milk was unaffected by the thermal processes tested. Therefore, the cortisol concentrations in commercial milk are expected to be similar to those in fresh milk products. This suggests that the stress indicator cortisol, remains stable during common heat processes in dairy milk products. Moreover, monitoring cortisol residues in processed milk products could be an alternative indicator to improve the quality of milk and mitigate cattle stress if raw milk is unavailable.