Introduction
The demand for meat has increased along with rapid population growth. Growing concerns about the environmental impact of raising and managing livestock have led to the need for alternative meat production methods (Henchion et al., 2017; Stephens et al., 2018). The United Nations has projected that the world’s population will reach 9.5 billion by 2050, which will double the demand for animal-derived proteins, thereby raising concerns about food sustainability (Population Reference Bureau [PRB], 2020). To address these concerns, several protein resources are being developed, including plant-derived proteins, insect-derived proteins, and in vitro meat (Henchion et al., 2017; Post, 2012). Among the future protein resources, in vitro meat, also known as cultured meat, cell-cultured meat, or clean meat, is edible and obtained by harvesting cells from living animals and multiplying them using cell engineering technology. Hence, this is a cellular agricultural branch that produces meat without raising livestock (Stephens et al., 2018). The potential of cultured meat as an important protein resource in foodborne illness prevention, environmental protection, animal welfare, and food crises alleviation is being explored (Goodwin and Shoulders, 2013). To produce cultured meat, three-dimensional (3D) bioprinting technologies that shape cell cultures into desired shapes and adjust the proportions of various components of cultured meat are key (Yang et al., 2024). This allows for the regulation of protein, fat, and other nutritional components and the creation of realistic edible meat (Handral et al., 2022).
3D bioprinting is a technology used to manufacture 3D biological structures by placing bioinks layer-by-layer. The technology has applications in organ transplantation, regenerative medicine, tissue engineering, and functional food production (Ozbolat et al., 2016). 3D bioprinting of cultured meat has the advantage of regulating the specific nutritional composition of the product, utilizing a variety of printing materials and by-products, and reducing waste (Bedoya et al., 2022). Although conventional two-dimensional (2D) culture technology only forms a 2D monolayer of cells, requiring incorporation of additional muscle fibers and adipocytes, 3D bioprinting technology easily produces relatively large-sized muscle tissue and complex structures through sophisticated arrangement of cell-containing bioinks and scaffolds and provides a more accurate in vivo-like environment than that of 2D culture (Guan et al., 2021; Lee et al., 2024). As 3D bioprinting technology in cultured meat has developed, bioinks have been established as an important material for cell insertion and maintenance of an appropriate environment (Veiga et al., 2021). Cultured meat bioinks are mainly made of naturally derived materials, most of which have viscoelastic properties and are produced through the printer nozzle (Wu et al., 2024). Therefore, selecting the correct bioink and setting the correct output ratio for 3D bioprinting is of great importance (Li et al., 2021).
As a key component of 3D bioprinting, bioinks transport cells and scaffold structures, and their biocompatibility, viscosity, precision, scaffold stability, and nontoxicity are important considerations (Li et al., 2021). Bioinks typically comprise hydrogel pre-polymer solution and cells; the hydrogel is in direct contact with the cells and forms scaffolds and contributes to bioink chemical and physical properties (Mandrycky et al., 2016). Hydrogels are broadly divided into synthetic polymer-based hydrogels, which are prepared by chemical synthesis, and natural polymer-based hydrogels (Zorlutuna et al., 2012). Synthetic polymer hydrogels, such as polyethylene glycol and polycaprolactone, stabilize scaffolds and provide accurate output; however, they are more expensive than natural polymer hydrogels and have poor biocompatibility, which is important for the survival and growth of cells (Bian, 2020). In contrast, natural polymer hydrogels, such as collagen and gelatin, mimic existing cell substrates and have excellent biocompatibility, which is favorable for cell motility, proliferation, and differentiation in cultured meat production (Carrow et al., 2015). Natural polymer hydrogels are classified into plant, marine, and animal hydrogels. Thus, in this study, we investigated the physiological features, advantages, and disadvantages of each hydrogel to select the most appropriate natural bioinks for cultured meat production and to subsequently use them in 3D bioprinting.
Animal-Based Bioinks
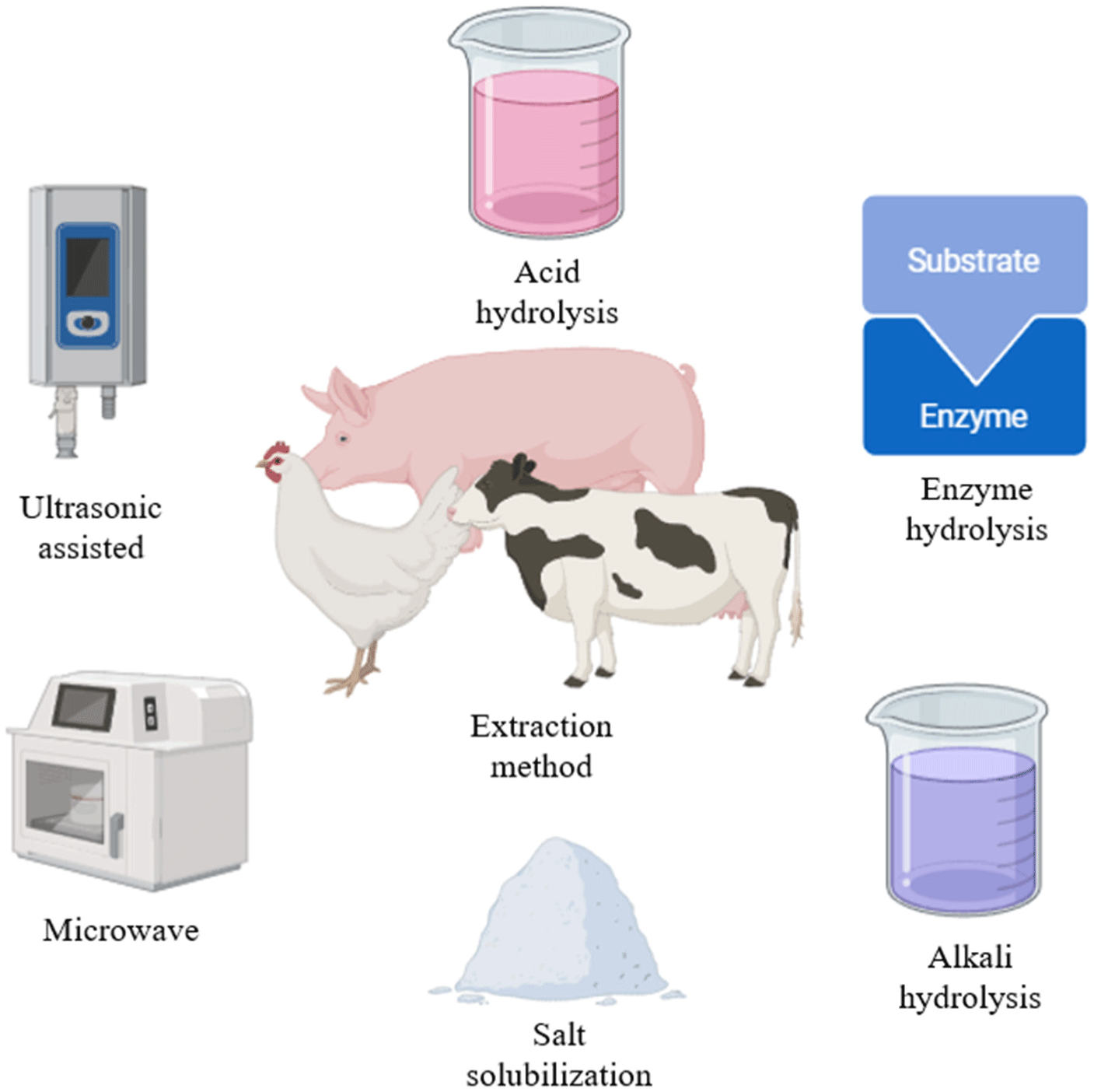
Animal-based bioinks are used in organ transplantation, regenerative medicine, and other applications, which have positive implications for cultured meat production. In meat production, skeletal muscles, which include muscle fibers along with connective tissue and intramuscular adipose tissue, are the main focus (Ramachandraiah, 2021). Animal-based bioinks are suitable for the growth of muscle satellite cells, as they most closely resemble natural cell physiological properties (Lu et al., 2022a). A popular animal-based bioink is collagen, which is a naturally occurring protein with bovine, porcine, and other animal origins. The protein has been extensively studied, has a high potential for success, and has excellent biocompatibility and low immunogenicity, thereby providing a suitable environment for cell growth and differentiation (Osidak et al., 2020). However, collagen has a high water content and low cross-linking level; hence, it is susceptible to deformity, resulting in an unstable scaffold structure that is difficult to maintain for a long period of time during bioprinting. Low-concentration collagen is limited in that it can only print planar structures up to 1–2 mm high owing to its low thermal stability. To solve these problems, studies on high-concentration collagen scaffolds are ongoing. However, an excessively high concentration of collagen also results in scaffolds that lack uniformity and inhibits cell proliferation and differentiation. Therefore, determining the appropriate collagen concentration that can maintain stable scaffolds while favoring cell survival is essential (Stepanovska et al., 2021b). Rhee et al. (2016) showed that maintaining the scaffold shape after printing was difficult when low collagen bioink concentrations (1–3 mg/mL) were used. However, when high collagen bioink concentrations (10–20 mg/mL) were used, a positive relationship between the concentration and the elastic modulus of the printed scaffolds was confirmed without affecting cell viability. In particular, cell viability and scaffold stability were maintained for 10 days, and the geometric accuracy of structures printed with 15 mg/mL and 17.5 mg/mL collagen solutions was reported to be 74%–78%. In addition, Stepanovska et al. (2021b) reported that collagen concentration and printability are positively correlated, regardless of cell viability, and that stable collagen scaffold printing is possible through parameters such as bioink temperature and appropriate printing conditions. Animal-derived collagen bioinks are widely used and studied in 3D bioprinting and have a high potential for success. Their advantages include excellent biocompatibility and low immunogenicity to maintain stable cell growth and differentiation. However, owing to their low viscosity, issues regarding scaffold stability scaffolds, printability, and mechanical synthesis exist, and further comprehensive research regarding the appropriate collagen concentration for 3D bioprinting is required (Fig. 1; Lu et al., 2022b).
Animal gelatin, which is mainly extracted from pig skin or bone by acetic acid pretreatment, heating, filtration, and drying, can be obtained by collagen hydrolysis. The protein has high cell adhesion, biocompatibility, and biodegradability, and is widely used as a bioink in cultured meat production (Kantono et al., 2022). Based on the manufacturing process, gelatin is divided into type A gelatin and type B gelatin. Type A gelatin is mainly obtained by acid treatment of collagen obtained from pigs, which is characterized by faster production than that of type B gelatin because it uses acid and has less cross-linking (Lu et al., 2022a). Type B gelatin, which is mainly obtained by alkaline treatment of bovine collagen, is characterized by high cross-linking compared with that of type A gelatin, which requires a longer manufacturing process but has high viscosity due to strong alkalinity (Lu et al., 2022b). Gelatin contains natural cell bonds, such as arginyl-glycyl-aspartic acid (RGD peptide), which promote cell adhesion, proliferation, migration, and differentiation, and is cheaper than collagen (Dutta et al., 2021). However, pure gelatin has poor mechanical compatibility for 3D bioprinting and has low thermal stability because gelatin hydrogen bonds cleave and dissolve at temperatures above 37°C. Therefore, to enhance the stability of the 3D structure and improve the printability, the implementation of a cross-linking process is essential (Kabiri et al., 2011). Asim et al. (2023) reported that the use of gelatin methacryloyl (GelMA) to stabilize gelatin scaffolds rendered them photocrosslinkable and suitable for 3D bioprinting. This enabled precise fabrication of various structures including cells. Initially introduced by Van Den Bulcke et al. (2000), GelMA is synthesized through the reaction between gelatin and methacrylic anhydride (MA), wherein the amino groups in gelatin are substituted with methacryloyl groups, producing a modified form of gelatin. Due to its retention of RGD sequences, robust thermal stability, and adaptable physical and chemical properties, GelMA hydrogels are widely applied in cell culture and tissue engineering (Sun et al., 2018). Therefore, animal gelatin bioinks have high biocompatibility and cell adhesion, and the thermal stability and mechanical compatibility problems can be remedied by gelatin modifications such as GelMA. The by-products of animals can be extracted and used to reduce negative environmental impacts by utilizing waste and resources from the conventional animal breeding and slaughtering process to ensure a steady supply (Noble et al., 2024). Furthermore, animal gelatin is a suitable 3D bioprinting bioink for cultured meat production at a lower cost than that using collagen bioink (Table 1).
| Scaffold materials | Target cell | Application | Reference |
|---|---|---|---|
| Porcine gelatin | BMSCs | Development of animal gelatin bioink scaffold for long-term stable cell culture | Li et al. (2021) |
| BEFS | Cultured meat scaffold study using animal gelatin bioinks to improve printability | Jeong et al. (2022) | |
| C2C12 | Potential for culturing mature root canals with a morphology similar to existing root canals when cells are cultured in animal gelatin hydrogel | Denes et al. (2019) | |
| C2C12 and 3T3-L1 | Potential for developing fat-containing cultured meat via porcine gelatin bioinks | Li et al. (2022a) | |
| Porcine collagen | pADSCs | Higher concentrations of animal collagen bioinks can overcome mechanical synthesis challenges | Stepanovska et al. (2021a) |
| Developing a high concentration of collagen bioink scaffold that does not negatively impact cell growth and differentiation | Matejkova et al. (2024) | ||
| MG63 and hASCs | Improving cell viability with animal collagen bioinks scaffold structure research | Yeo et al. (2016) | |
| RbAC | Research on fabricating scaffolds using a blend of cell and animal collagen bioinks | Koo et al. (2018) | |
| Rat cartilage cells | Evaluating cell compatibility using high concentration animal collagen bioink scaffolds | Isaeva et al. (2021) | |
| MG63 and hASCs | Develop porous, biocompatible scaffolds with animal collagen bioinks | Kim et al. (2016) | |
| L929 | Stability and cell viability of porcine collagen bioink scaffolds studied | Maher et al. (2022) | |
| C2C12 | Development of aligned collagen fiber bundle scaffolds for efficient cell differentiation | Kim and Kim (2019) | |
| C2C12 and hESC-CM | Development of cultured meat scaffolds using SPI, PPI and polysaccharide hydrogel bioinks | Lee et al. (2019) | |
| Bovine gelatin | L6 rat myoblasts | Development of cytocompatible and mechanocompatible scaffolds with bovine gelatin bioinks | Suvarnapathaki et al. (2019) |
| Porcine gelatin and bovine gelatin | C2C12 | Researchers develop edible cultured meat scaffold using bioink mixed with animal gelatin and chitosan | Li et al. (2022b) |
Animal-based bioinks, such as collagen and gelatin, are the most commonly used in cultured meat production. These cells differentiate into cell types typically associated with meat, and in cultured meat production, they proliferate and differentiate into fibroblasts such as skeletal muscle cells (Reiss et al., 2021). Bryant and Barnett (2020) found that consumers have ingredient and nutritional concerns about plant-based proteins and prefer animal protein. Furthermore, cultured meat produced from alternative proteins, such as insect or plant protein sources, is less palatable because it does not resemble meat from conventional livestock. Animal-based bioinks provide the right extracellular matrix (ECM) for cell survival and growth, produce cultured meat with texture and nutritional properties similar to that of conventional meat, and provide a continuous supply of familiar meat without the need for slaughter. Animal-based bioinks have the advantage of forming biocompatible scaffolds that effectively deliver nutrients suitable for cell proliferation, thereby allowing them to mature into edible meat products (Reiss et al., 2021). In addition, 60% of the waste generated by the meat industry is currently cattle and porcine sources, and traditional waste disposal methods such as incineration and burial cause environmental problems, so research is being conducted to convert animal-based bioinks used in bioprinting (Shibru et al., 2024). It is believed that this method can achieve sustainability and cost-effectiveness through waste recycling. In addition, animal protein can be produced without mass slaughtering, which has a positive impact on animal welfare and appeals to ethical consumers (Soleymani et al., 2024). Animal-based bioinks for 3D bioprinting are being explored by extracting muscle cells from various livestock species; however, they are yet to reach the scale and costs required for commercial mass production and sale of cultured meat. Therefore, further research is needed to develop the most suitable animal-based bioinks for cultured meat production and ensure machine stability.
Plant-Based Bioinks
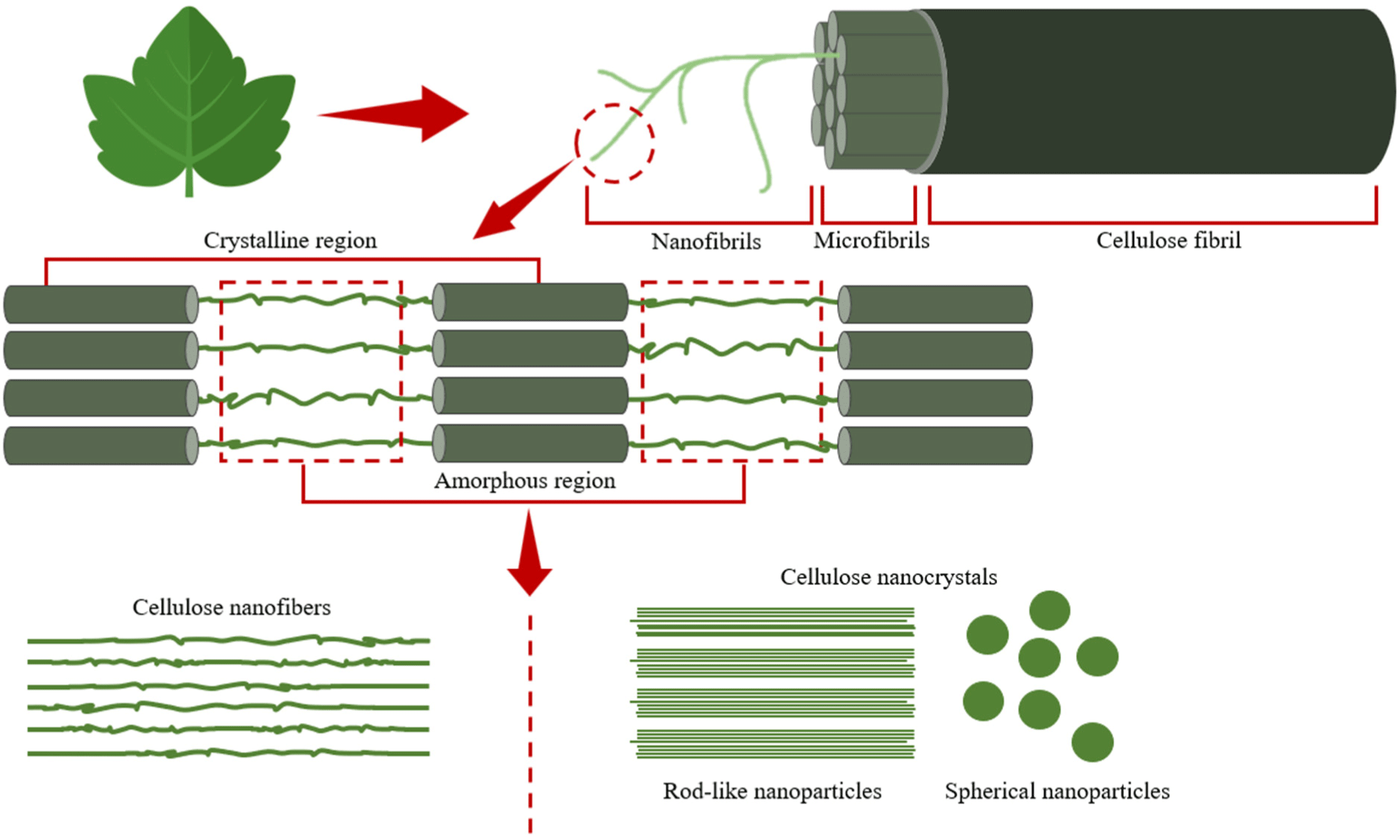
Plant-based bioinks are renewable and biodegradable, which minimizes their environmental impact, and are also an inexpensive and abundant source of protein, which is important for the development of sustainable bioprinting technologies. Among the most commonly utilized plant-based bioinks, cellulose is one of the most widely distributed natural polymer sources in nature. Cellulose is the main structural element of plant tissue cell walls and is present in fruits, trees, plants, leaves, and bark (Fatimi et al., 2022). Nanocellulose, which is made by breaking down cellulose into nanometer-sized fibers or crystals, biodegradability, and biocompatibility and is used in bioprinting due to its high viscosity and gel-forming ability (Armstrong et al., 2022). Guo et al. (2023) reported that nanocellulose-based bioinks stack cells and form support structures to produce functional cultured meat; hence, they are considered a suitable material for cultured meat production. However, despite its high mechanical strength due to its nanometer-sized fibers, setting precise printing parameters, such as the injection pressure and printing temperature, is difficult. In addition, nanocellulose has a low zeta potential on its surface, rendering it more viscous (Ee and Li, 2021). This not only increases the likelihood of agglomeration in nozzle-based bioprinting, which clogs the nozzle, but also negatively affects cell growth depending on the structure and composition of the bioink (Han et al., 2020). Moreover, cells may not be evenly distributed in the deep interior of the scaffolds, which requires further investigation (Han et al., 2020). Bioinks are produced by mixing with water-soluble substances to reduce the high viscosity, but nanocellulose is highly hydrophilic, which makes it unprintable when mixed, and it is known that double cross-linking is required to prevent this (Ajdary et al., 2019). However, the crosslinking agents required for double crosslinking are mainly glutaraldehyde or genipin, which are toxic and require pretreatment or purification (Dobaj Štiglic et al., 2023). To solve these problems, research is being conducted on fine-tuning the concentration of nanocellulose bioinks and using physical crosslinking or UV curing rather than chemical crosslinking (Wei et al., 2021). Physical crosslinking is a method that uses ions such as calcium ions (Ca2+) to stabilize nanocellulose fibers, which has the advantage of lower cytotoxicity risk and better biocompatibility than chemical crosslinkers (Monfared et al., 2021). UV curing is a method of curing with a photocurable material, which can improve mechanical strength and rapidly anchor precise structures (Tang et al., 2018). In the study of 3D printing nanocellulose supports for mechanical stability by Xu et al. (2018), double crosslinking, including ionic crosslinking, was performed to print supports with improved mechanical stability. Nanocellulose-based bioinks are inexpensive, readily available, and highly viscous; hence, they are favorable for stable scaffolds and cell attachment in cultured meat production. However, their high viscosity may result in nozzle clogging issues in 3D bioprinters, which hinders the continuity and accuracy of printing and negatively affects cell growth (Wang et al., 2020). Therefore, continuous research and development on the optimal nanocellulose concentration in bioprinting and detailed printing parameters for 3D bioprinters is necessary (Fig. 2; Wan Jusoh et al., 2022).
Bean protein, such as soy protein isolate (SPI) from soybeans and pea protein isolate (PPI) from peas, is a low-cost and abundant source of protein with functional and physicochemical properties that make it a viable alternative to animal-derived protein sources (Ianovici et al., 2022). In the food industry, soy protein has been widely studied as a substance that mimics conventional meat and has the advantages of being hypoallergenic and highly nutritious. Soy proteins in cultured meat are processed into various forms; hence, they are highly biocompatible and provide an environment conducive to cell attachment and growth. Moreover, they are generally well accepted by the immune system and have low immunogenicity (Singh et al., 2023). David et al. (2024) fabricated cultured meat scaffolds using pea protein and found that scaffolds fabricated via 3D bioprinting from a mixture of PPI- and RGD-modified alginate supported the myogenesis of bovine satellite cells. Sharma et al. (2023) also reported that isolated soy protein bioinks are environmentally friendly when used in cultured meat production. In addition, PPI bioinks have low solubility and water retention, which reduces the printing precision of 3D bioprinted scaffolds, and SPI bioinks also require comprehensive studies on printing parameters, such as printing temperature, printing speed, and injection pressure, to ensure the stability of the scaffolds (Chen et al., 2024). To address these issues, blending with polymeric mixtures such as alginate or gelatin to complement the mechanical strength and improve the structural stability of the support has been studied (Carranza et al., 2024). In a study on the development of hydrogels blended with SPI and alginate for tissue engineering by Alesaeidi et al. (2023), it was reported that blending SPI and alginate improved the viscosity of the hydrogel, enhancing its mechanical strength and forming a stable support. The study of soy protein and agar residue for 3D printing by Uranga et al. (2023) also reported that blending agar residue with soy protein improved mechanical performance and produced stable structures. Therefore, among plant-based bioinks, bean protein isolate bioinks, such as PPI and SPI, have the advantages of low cost, rich nutritional value, and favorable cell adhesion. Many studies have been conducted to produce cultured meat as a representative animal protein substitute. However, there is a problem of poor mechanical stability, so research on the development of composite hydrogels with polymer mixtures to compensate for this continues, and it is considered necessary to develop hybrid bioinks based on SPI (Table 2).
| Scaffold materials | Application | Reference |
|---|---|---|
| SPI | Research on the development of 3D bioprinted edible scaffolds using SPI bioinks | Takemasa (2021) |
| Developing culture meat scaffolds and studying cell adhesion using SPI bioinks | Mariano et al. (2024) | |
| Improving printability by developing SPI bioink scaffolds and improving ink density | Carranza et al. (2024) | |
| Developing scaffolds for three-dimensional cell culture using SPI bioinks | Ma et al. (2024a) | |
| Improving printability and structural texture in 3D bioprinting by blending SPI bioinks with multiple polyphenols | Mohammadi et al. (2023) | |
| PPI | Study of BSc cell differentiation on culture meat scaffolds printed with PPI bioinks | David et al. (2024) |
| Research to develop long-term stable culture meat scaffolds using PPI bioinks | Ianovici et al. (2024) | |
| Research on improving printability of 3D bioprinting by mixing PPI bioinks with sodium alginate | Ma et al. (2024b) | |
| Adjusting the proper water content of PPI bioinks to improve printability in 3D bioprinting | Venkatachalam et al. (2023) | |
| Research on 3D bioprinted hydrogels with PPI bioinks | Chen et al. (2024) | |
| SPI and PPI | Characterization and cell adhesion of cultured meat scaffolds injected with PPI and SPI bioinks | Kim et al. (2024) |
| Development of cultured meat scaffolds using SPI, PPI, and polysaccharide hydrogel bioinks | Wollschlaeger et al. (2022) | |
| Research on the development of cultured meat scaffolds using SPI and PPI bioinks | Ianovici et al. (2022) | |
| SPI, wheat protein (WP), peanut protein (PP) | Cultured meat scaffold quality evaluation study using SPI and plant protein bioinks | Zheng et al. (2022) |
| SPI, wheat gluten (WG), rice protein (RP) | Research on developing a cultured meat scaffold by mixing SPI with other plant proteins | Qiu et al. (2023) |
| SPI, canola (CAPI), chickpeas (CHPI), potatoes | Developing a plant-based bioink culture meat scaffold by comparing SPI with various plant proteins | Israeli et al. (2023) |
| SPI, fibrous silk fibroin (SF) | Cell culture on scaffolds containing protein tertiary structures with SPI bioinks and SFs | Dorishetty et al. (2021) |
Plant-based bioinks are the most researched bioinks after animal-based bioinks because they use less water and produce less area than that of animal-based bioinks during the raw material production process. This approach minimizes resource consumption, enhancing cost efficiency and lowering production expenses in large-scale manufacturing. In addition, scaffolds made from plant-based bioinks have hydrophilicity, low immunogenicity, and good nutritional content, which are important for cell growth. Moreover, plant-based bioinks have good biodegradability, which minimizes the negative impact on the environment, reduces the problem of waste after cultured meat production, and contributes to sustainable production (van Vliet et al., 2020). However, cultured meat produced with plant-based bioinks has a different flavor and texture than that of cultured meat produced with animal-based bioinks. Cultured meat using animal-based bioinks is characterized by mature myofibrils and bundles of a certain thickness and length that are transformed into skeletal muscle tissue after cultivation. This tissues is similar to the skeletal muscle tissue of animal meat and has a texture similar to that of conventional animal meat in terms of elasticity. However; plant-based bioinks lack elasticity due to their loose fiber structure and lack texture and are bitter owing to the compounds in the raw plant materials (Wang et al., 2023). To address these challenges, researchers are exploring methods to replicate the taste of meat by incorporating flavor precursors such as thiamine, as well as enhancing texture to mimic meat through technologies like thermoplastic extrusion of soy protein tissue (Milani et al., 2022). In addition, plant-based bioinks, which mainly comprise polysaccharides and proteins, have a simpler structure than that of animal-based bioinks, resulting in lower mechanical compatibility owing to the lack of intermolecular interactions compared with that of animal-based bioinks. Thus, plant-based bioinks and the printed scaffolds are deformed by external forces, or the structures are damaged and weakened over time, negatively affecting the function of the cultured cells (Padhi et al., 2023). Therefore, although plant-based bioinks are a low-cost, sustainable, and eco-friendly raw material, optimization for consumer acceptance is needed. This includes using cross-linking agents to strengthen the bonds between proteins to improve texture, taste, and appearance to resemble that of conventional animal meat, and improvement of 3D bioprinter machine compatibility to maintain stable output and scaffolds. Hence, many aspects of bioinks require improvement for commercialization of cultured meat.
Marine-Based Bioinks
Materials derived from marine resources have gained attention as favorable bioinks for cultured meat production due to the absence of religious restrictions associated with the use of marine resources (Zhang et al., 2018). The most representative marine-based bioink is fish gelatin, which can be obtained from marine resources, such as fish skin, bones, and fins (Karim and Bhat, 2009). Effectively utilizing the main by-products of the fish processing industry, which cause waste and pollution, prevents environmental problems when manufacturing gelatin (Badii and Howell, 2006). In addition, fish gelatin has low toxicity; hence it does not have harmful effects on cells, and it is eco-friendly, biodegradable, and biocompatible; thus, it promotes the growth of cells and printed tissues (Maihemuti et al., 2023). Lee et al. (2022) revealed that fish gelatin is less stable than mammalian gelatin due to the lower hydroxyproline (Hyp) and proline (Pro) content in the amino acid sequence, which influences the gelatin structure and properties. The lower the Hyp and Pro content, the lower the gelatin gel strength and melting point. In particular, fish gelatin properties are greatly affected by the pH, temperature, pretreatment, extraction process conditions, and the type of raw fish. Thus, producing gelatin with consistent properties is difficult. Therefore, establishing technologies to improve fish gelatin functional properties is necessary (Huang et al., 2019). In addition, fish gelatin has a lower melting point compared to animal gelatin due to its adaptation to marine temperatures, which makes it easily deformed at high temperatures and has a high water absorption rate, resulting in poor mechanical stability (Alfaro et al., 2015). In a study on the development of cold-water fish GelMA hydrogels for tissue engineering, Yoon et al. (2016) observed that fish-derived GelMA hydrogels exhibited higher water absorption and faster degradation rates compared to porcine GelMA hydrogels, and reported that further research is required to improve long-term mechanical stability. Fish-gelatin bioinks also carry the risk of allergic reactions depending on the type of raw material (Mukasheva et al., 2024). Wang et al. (2024) showed that rats fed scaffolds injected with pollock fish gelatin exhibited intestinal wall damage, mast cell degranulation, and high allergic reactions. Thus, further research is needed to reduce the allergic risk of fish gelatin. A study by Wang et al. (2024) on allergenicity and digestive resistance linear epitopes in fish gelatin for cultured meat cells reported that the protein structure of fish gelatin may be recognized as a threat by the immune system and cause allergic reactions, and that digestive resistance linear epitopes in fish gelatin can bind to immunoglobulin E (lgE) antibodies and induce allergic reactions. It was reported that gelatin extracted from cod showed higher allergic reactions compared to other fish species, and that allergic reactions may differ depending on the protein structure of the fish species, so further studies are needed depending on the fish species (Wang et al., 2024). Therefore, although fish gelatin bioinks have the advantages of being eco-friendly and having excellent biocompatibility that is favorable for cell growth and differentiation, they lack mechanical stability due to low melting point and high water absorption, and have the risk of causing allergies depending on the fish species, so further studies are needed to solve these problems for long-term cell culture such as cultured meat (Table 3).
| Scaffold materials | Application | Reference |
|---|---|---|
| Salmon | Improving cross-linking of fish gelatin bioink scaffolds for cell differentiation | Acevedo et al. (2020) |
| Development of aligned nanofiber fish gelatin bioink scaffolds for mimicking extracellular matrix | Taborda et al. (2023) | |
| Evaluating the printability of salmon gelatin bioinks for 3D bioprinted foods | Carvajal-Mena et al. (2022) | |
| Lizardfish | Fish scale gelatin extraction and hydrogel injection for 3D bioprinting | Pasanaphong et al. (2024) |
| Study of physical properties and biocompatibility of fish gelatin bioink scaffolds | Boonyagul et al. (2022) | |
| Tilapia | Myoblast differentiation potential of scaffolds injected with fish gelatin bioink | Shi et al. (2022) |
| Triggerfish | Rheological characterization of fish gelatin hydrogels extracted using ultrasonic technology | Ahmad et al. (2024) |
| Tilapia, flounder, cod | Evaluation of skin cell activity of gelatin bioink scaffolds derived from different species of fish | Lee et al. (2023) |
| Tilapias, pangasius, cod | Allergenicity of fish gelatin bioink culture meat scaffold | Wang et al. (2024) |
| Cold-water fish | Tissue compatibility study of scaffolds using fish gelatin bioinks | Maihemuti et al. (2023) |
| Development and potential of fish gelatin for use as a bioink | Yoon et al. (2016) | |
| Rheological properties of fish gelatin hydrogels blended with alginate | Derkach et al. (2021) | |
| Cell adhesion and proliferation on scaffolds injected with fish gelatin bioink | Gomes et al. (2013) | |
| Developing a 3D bioprinting scaffold using a blend of fish gelatin bioink and cells | Yu et al. (2020) | |
| Modulating the pore size of fish gelatin bioink scaffolds to enhance cell survival in scaffold development | Toader et al. (2023) |
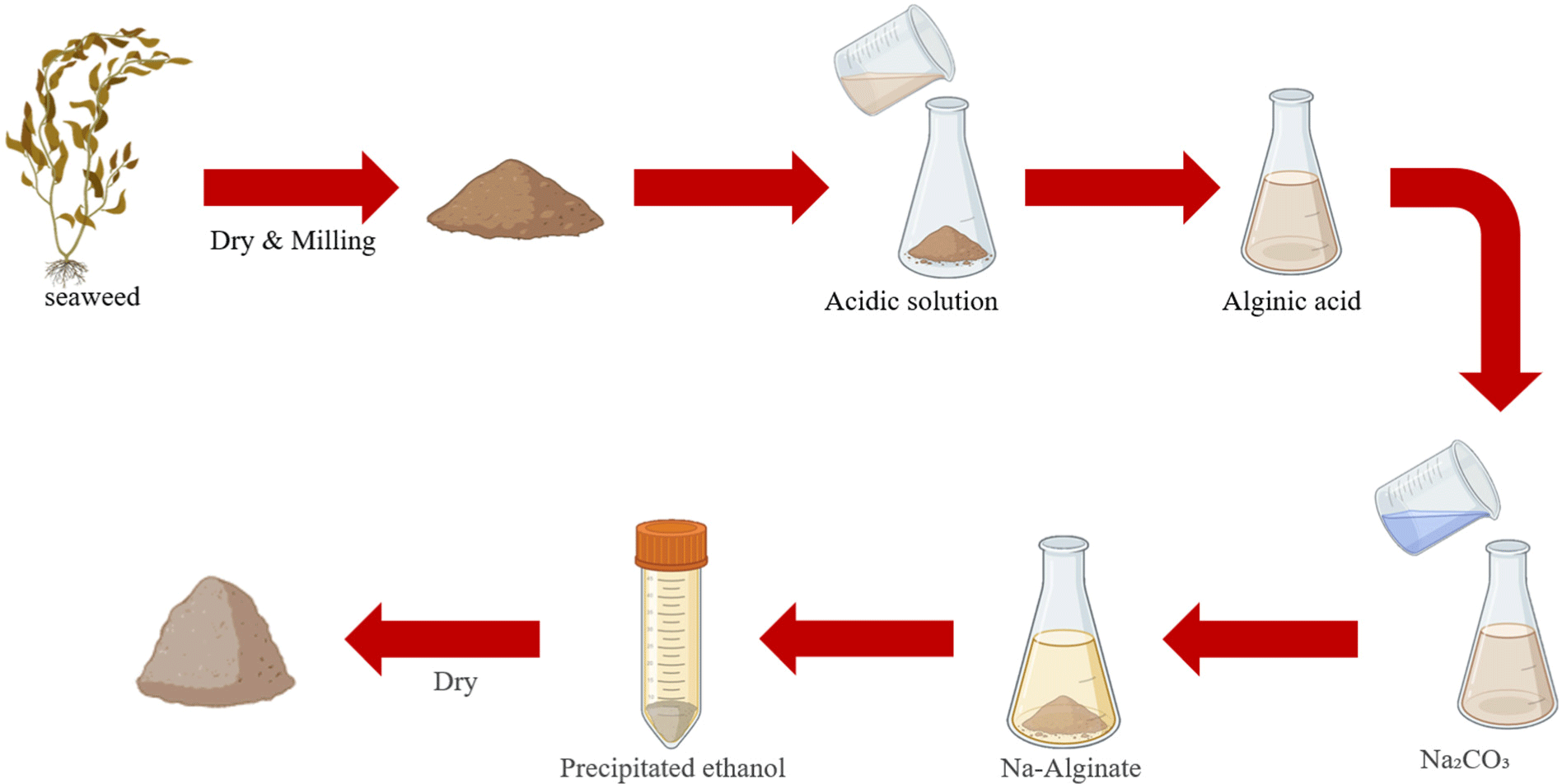
Alginate is a natural marine polysaccharide bioink and a non-animal-derived material that is mainly extracted from the cell walls of algal cells such as brown algae (Lin et al., 2023). Currently, alginate is mainly used in regenerative medicine, such as tissue engineering, bone regeneration, and wound healing, and has advantages such as biodegradability and biocompatibility (Gao et al., 2021). In addition, because alginate is non-cytotoxic, edible, and relatively inexpensive, it is often used in binders and stabilizers in food science, such as in cultured meat production (Lee et al., 2024). Scaffolds produced with alginate bioinks were not suitable for printing complex structures because of their low mechanical compatibility, and the scaffolds did not remain stable for long periods of time (Li et al., 2016). In addition, low mechanical compatibility does not provide a stable environment for cell attachment and growth, resulting in low cell survival (Gao et al., 2021). Furthermore, alginate, which is composed of two main components with hydrophilic components, mannuronic acid (M) and glucuronic acid (G), lacks the formation of hydrophobic surfaces for cells to attach to, and does not contain cell adhesion sequences such as RGD, which binds to integrin receptors on the cell surface and allows cells to attach to the substrate, thus preventing cells from adhering naturally (Rahman et al., 2024). To solve this problem, research is underway to enhance cell adhesion by mixing cell adhesion sequences such as RGD peptides with alginates or with gelatin or collagen, which are materials that increase biocompatibility. In a study of RGD peptide-modified alginates for tissue engineering applications, Sandvig et al. (2015) observed that muscle cells survived for up to 41 days on alginates mixed with RGD peptides and reported that alginates mixed with RGD peptides can enhance cell adhesion. However, alginate has an irregular biodegradation rate, which negatively affects the growth and differentiation of cells in cell engineering, such as in cultured meat, and alters bioink mechanical and biological properties (Axpe and Oyen, 2016). To address this, research is underway to modulate the mechanical properties and degradation rate, and a study by Tahir and Floreani (2022) on double cross-linked alginate-based hydrogels for cultured meat, reported that double cross-linking via ionic cross-linking and photocross-linking allows muscle satellite cells to grow stably on cross-linked alginate. It was reported that double cross-linking enhances the mechanical strength of alginate hydrogels, and the support remains stable, which may have a positive effect on cell survival (Tahir and Floreani, 2022). Collectively, the marine-based bioink alginate is biocompatible, non-cytotoxic, and safe; however, using it alone is difficult because of its low printability and difficulty in maintaining scaffold stability. Therefore, other hydrogels or cell adhesion peptides must be added. In addition, the alginate’s irregular rate of biodegradation requires further research (Fig. 3).
The ocean represents a renewable resource, making marine resource utilization a promising approach to addressing environmental pollution and energy shortages. Therefore, continuous research on marine resources has revealed many compounds that have been isolated from marine organisms and used as materials for biomedical applications such as cell culture and regenerative medicine (Silva et al., 2012). Marine resources impose no regulatory or religious restrictions on mammals, are biodegradable and biocompatible, and can be used as scaffold materials in tissue cell cultures (Zhang et al., 2022). Fish gelatin bioink is produced from about 50%–70% of by-products, including fish scales, bones, and viscera, and is a new alternative biological material derived from underutilized marine food waste, which is a protein-rich resource. Owing to its low cost and similar properties to those of mammalian gelatin, the use of fish gelatin can increase its economic value and reduce waste problems that negatively impact the environment (Boonyagul et al., 2022). In addition, alginate, a natural polysaccharide extracted mainly from brown algae, has excellent biocompatibility, non-immunogenicity, and biodegradability, rendering it cell-friendly, and has been utilized as a 3D bioink for meat cell culture scaffolds. However, most marine-based bioinks, such as fish gelatin and alginate, have poor mechanical compatibility, which easily deforms or collapses the scaffolds after printing, and are easily damaged and degraded in the external environment; hence, creating stable scaffolds is difficult (Züger et al., 2023). To solve this problem, Hong et al. (2015) used polyethylene glycol, a synthetic polymer produced by the polymerization of oxide that can adjust its viscosity according to different molecular weights, to improve alginate with low mechanical compatibility, adjust the rheological properties of the bioink, and construct a bioink with high strength and biocompatibility. Jeevithan et al. (2013) also used fish gelatin bioinks with chitosan and calcium salts to minimize the deformation of gelatin, maintain scaffold stability, and promote cell growth, resulting in stable scaffolds. Therefore, various blends and additives are being explored to improve the physical properties and mechanical compatibility of marine-based bioinks. Although marine-based bioinks with high biocompatibility create a cell-friendly environment that favors cell proliferation and differentiation, they are not universal for different cell types. This negatively affects cell adhesion and growth in certain cell types; hence, they need to be blended with other appropriate bioink components for improvement (Bomkamp et al., 2022). In addition, most marine resource feedstocks are not market-oriented because of the lack of regulations regarding extraction and purification on an industrial scale. Depending on the feedstock, there is a risk of allergic reactions in consumers, which will require an ample waiting period before commercialization (Silva et al., 2012). Overall, marine-based bioinks have potential as valuable bioinks that enable low-cost and high-quality 3D bioprinting for cultured meat production scaffolds; however, they are not suitable for large-scale production for industrial commercialization due to low mechanical compatibility and limited research on ink raw material extraction technology. Therefore, further regulation and research on various marine resources that are raw materials for marine-based bioinks are needed.
Conclusion
Cultured meat is one of the most promising protein alternatives for traditional animal-derived proteins, and cellular agriculture is a potential solution to this food crisis. Cell culture, which is important for meat production, requires the cells to be cultured in a suitable environment for growth and differentiation, which is closely related to bioinks. Bioink properties affect mechanical compatibility, structural stability of the scaffolds, biocompatibility of the cells, and nutrition of the cells. Bioinks can be animal, plant, marine, or other chemicals. However, animal-based bioinks are the most widely studied. Animal-based bioinks are highly preferred by consumers compared to other bioinks and have the advantage of providing an ECM suitable for cell survival and growth, thereby enabling the production of cultured meat that is similar to conventional meat. Typical animal-based bioinks include animal collagen and gelatin, which provide nutrients favorable for cell proliferation and form a biocompatible support, effectively providing an environment for cell maturation. However, animal collagen and gelatin suffer from a lack of mechanical compatibility, which stunts long-term maintenance of scaffold stability. To overcome this limitation, the development of GelMA with higher gelatin concentrations and the incorporation of cross-linking agents is underway. GelMA hydrogels can improve the mechanical compatibility of bioprinting by ensuring that the RGD sequence is retained, and have excellent thermal stability and flexible physical and chemical property tunability. Vegetable bioinks have high hydrophilicity, which makes it difficult to form stable supports due to poor mechanical compatibility, and their raw material characteristics make it difficult to mimic the taste and texture of traditional meat. Marine bioinks are less mechanically stable due to their low melting point and high water absorption, and depending on the raw material, they can be allergenic. To produce cultured meat that has a texture and taste similar to that of conventional meat and is not rejected by consumers, animal-based bioinks are essential. Continuous research on animal-based bioinks, animal collagen, and gelatin bioink scaffolds are vital to commercialize cultured meat.