Introduction
Cell-based meat (CBM), also referred to as in vitro or lab-grown meat, represents a significant and innovative shift in sustainable and ethical cell-based food production. This distinctive procedure aims to address key challenges related to traditional meat production, such as maintaining food security, improving environmental sustainability, and enhancing animal welfare (Kim et al., 2023a). The increasingly unsustainable nature of conventional animal husbandry, characterized by significant environmental implications and ethical concerns, has resulted in an unprecedented demand for alternative protein sources (Bakhsh et al., 2022). CBM, produced utilizing cell culture technologies in controlled environments, offers a potential solution to the world’s growing protein demand while mitigating the negative impacts of traditional meat production (Balasubramanian et al., 2021; Hadi and Brightwell, 2021).
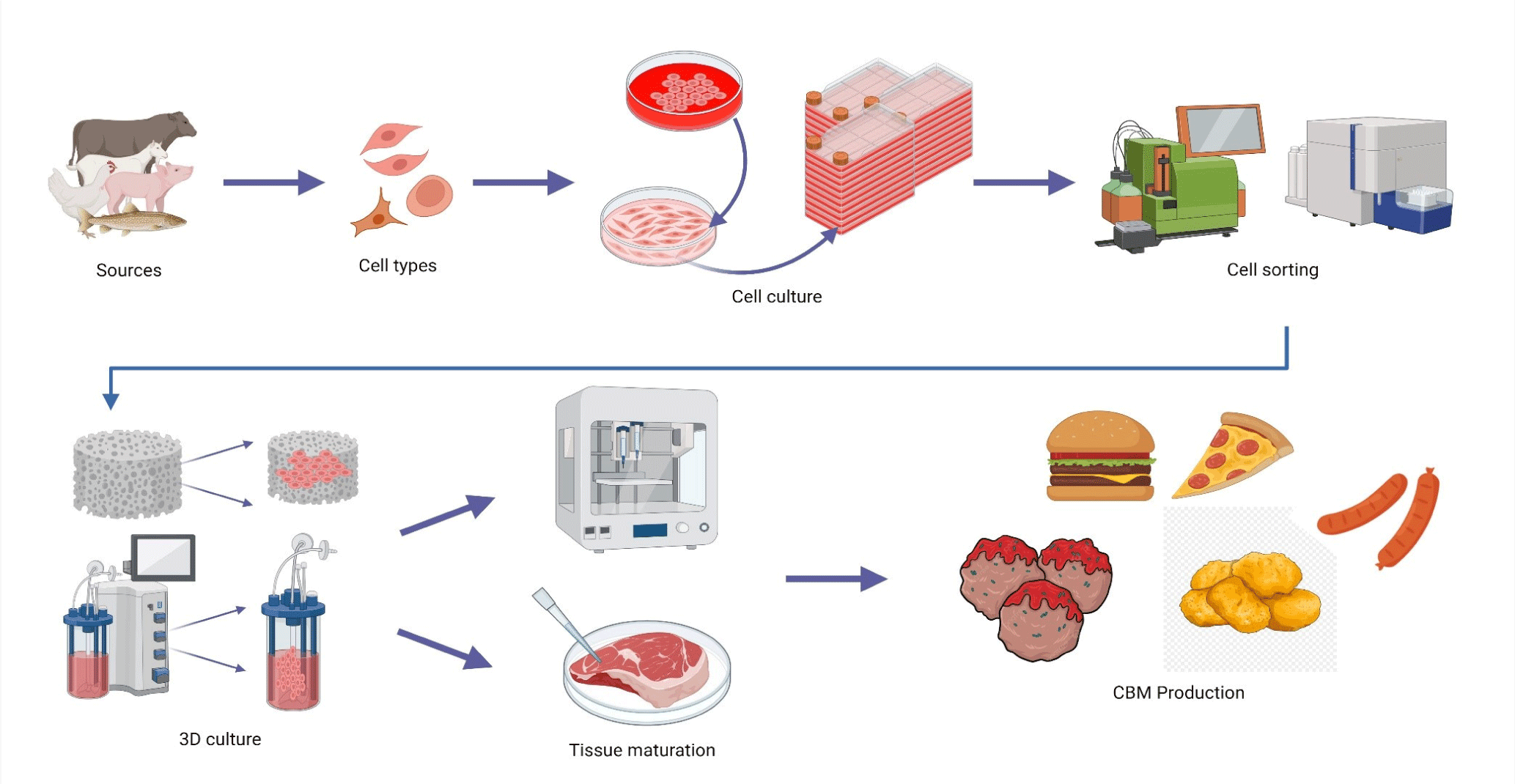
CBM production involves cultivating animal cells in a bioreactor and supplying the substrate with adequate nutrients and growth factors that facilitate their multiplication and transformation into muscle tissue (Fig. 1). This approach not only eliminates the need to slaughter animals but also possesses the potential to significantly reduce greenhouse gas emissions, land use, and water usage (Joo et al., 2022). Studies have demonstrated that CBM production potentially reduces land use, water use, and greenhouse gas emissions by up to 99%, 96%, and 96%, respectively, compared with traditional meat production methods (Bhat et al., 2019; Gaydhane et al., 2018; Munteanu et al., 2021). Furthermore, CBM addresses and minimizes public health risks associated with extensive livestock farming, such as zoonotic diseases and antibiotic resistance (Bernstein and Dutkiewicz, 2021; Gilbert et al., 2021).
Nonetheless, transitioning from an idea to a market-ready product requires successfully navigating a complex landscape of safety and regulatory concerns. For instance, conceivable safety hazards include possible viral, prion, and other pathogenic contamination and genetic engineering procedures that potentially introduce undesired risks (Ong et al., 2021). The distinctive components and procedures used in CBM manufacture necessitate rigorous safety assessments and the implementation of standardized testing protocols. The use of novel materials and techniques in CBM production warrants extensive safety valuations and the development of standardized testing methodologies (Kim et al., 2023b; Ong et al., 2021).
Similarly, the nutritional safety of CBM is a critical area of concern, as its production processes involve novel techniques that may influence its nutrient composition. Unlike conventional meat, the micronutrient profile of CBM, particularly concerning essential elements such as iron, vitamins, and fatty acids, remains an underexplored area that necessitates further research (Fraeye et al., 2020). However, as CBM has the potential to mitigate contamination risks associated with traditional livestock, such as pathogens, the rapid proliferation of cells in its production raises concerns about potential nutrient imbalances or unintended cellular behavior (Chriki and Hocquette, 2020). For instance, the integration of additives, such as hormones and growth factors, highlights the need for comprehensive safety assessments to ensure that lab-grown meat is nutritionally comparable to or superior to conventional meat (Fraeye et al., 2020). Furthermore, consumer confidence in the safety of CBM profoundly depends on transparent communication about its nutritional composition and strict adherence to rigorous safety standards and protocols (Bryant and Barnett, 2018).
Regulatory systems must be designed to address each of these complications, ensuring that CBM products satisfy stringent safety standards before being availed to consumers (Pontalti et al., 2024). Extensive safety assessments should cover the entire production process, including cell line selection, growth medium composition, bioreactor settings, and post-harvest processing. Regulatory systems should be customized to tackle these particular issues, ensuring that CBM products fulfill stringent safety standards before being distributed to consumers (Rubio et al., 2020). The regulatory framework for CBM is still in its early stages, with several countries employing different approaches for regulation and authorization. For example, the United States (US), through the Food and Drug Administration (FDA) and US Department of Agriculture (USDA), and the European Union are developing specialized regulatory pathways to ensure the safe introduction of CBM to the market (Vlčko et al., 2023).
Efficient regulatory procedures demand collaboration among researchers and regulatory organizations to formulate comprehensive guidelines that address aspects of CBM production. This includes examining source and residue safety, examining contamination potential, and developing non-animal safety assessment procedures (Cabral et al., 2024). This review aimed to identify potential safety hazards, including the presence of pathogens and the implications of genetic engineering. It emphasizes the necessity for comprehensive safety evaluations and standardized testing procedures. Additionally, the review addresses the evolving regulatory landscape, highlighting the efforts made and challenges encountered in establishing comprehensive standards.
Biological Safety
The initial step in CBM production involves sourcing animal cells, typically from muscle, fat, or connective tissue. Animal tissue extraction is a critical stage in CBM production, necessitating precise techniques to achieve optimal results (Kathera and Kim, 2024). The procedure commences with a biopsy from the donor animal, which is maintained under stringent aseptic conditions to prevent the introduction of pathogens, including bacteria, viruses, and fungi. Even minimal contamination can rapidly proliferate in cell cultures, leading to substantial challenges in maintaining the integrity and viability of the CBM production process. Effective screening and disinfection processes are requisite to mitigating the risk of zoonotic diseases, which can be transferred from animals to humans, further ensuring the safety and reliability of the CBM production system (Melzener et al., 2021). For instance, molecular diagnostic tools, such as polymerase chain reaction and next-generation sequencing, have enhanced pathogen detection and the ability to keep cell cultures free of contamination. The utilization of automated, closed-system biopsies can limit the danger of human error and contamination, hence improving the sterility of tissue extraction techniques (Sogore et al., 2024).
Moreover, the viability and quality of the extracted tissue are paramount for the effective generation of in vitro meat. To preserve cell viability, minimizing tissue damage during extraction, maintaining optimal tissue storage conditions, and rapidly treating the tissue are essential (Zidarič et al., 2020). Nevertheless, ethical considerations and animal welfare are critical when collecting tissue for CBM. Donor animal distress and discomfort can be considerably diminished by using compassionate and less invasive biopsy techniques. The development of non-invasive or minimally invasive sampling procedures, such as skin biopsies or fine-needle aspirations, can improve animal welfare (Campbell, 2019).
Guaranteeing sterility is of paramount importance when initially isolating cells to prevent any microbial contamination that could compromise the entire production process. Antibiotics, commonly used in cell culture media, can induce metabolic alterations in cells, potentially affecting experimental outcomes and cell line stability (Elliott and Jiang, 2019). Culture medium contamination poses a significant risk during cell proliferation and differentiation. Maintaining sterility and ensuring the absence of contaminants when performing medium changes are imperative. The stability and growth-promoting properties of culture media potentially degrade over time, affecting cell culture reliability (Table 1). Certain medium formulations have a 6-months stability period, while others lose effectiveness faster (Kuleshova et al., 2021).
| No. | Title | Authors | Focus | Key findings |
|---|---|---|---|---|
| 1 | Scale up economics for cultured meat | Humbird (2021) | Economic challenges and microbial risks in scaling cultured meat production | Scalability is limited by low growth rates, metabolic inefficiency, and cell damage. Meeting the target cost of ~$25/kg for bulk cell mass is essential, though perfusion processes exceed this cost. Plant hydrolysates may offer a solution but require further development. Enhancing metabolic efficiency and lowering media costs are crucial for displacing conventional meat. |
| 2 | Microbiological and chemical hazards in cultured meat and methods for their detection | Sogore et al. (2024) | Examination of microbial and chemical hazards in cultured meat production | Identified potential microbial and chemical contaminants at each stage of production. Emphasized the need for robust safety protocols, scalable testing methods, and specialized detection systems to monitor contaminants unique to cultured meat. Recommended implementing digital food safety technologies for real-time monitoring and regulation to enhance safety and consumer confidence. |
| 3 | Challenges and possibilities for bio-manufacturing cultured meat | Zhang et al. (2020) | Examination of technological and societal challenges in cultured meat production | Identified challenges including high production costs, lack of nutrients, and the need for food safety certification. Proposed solutions involve advancements in tissue and bioreactor engineering, synthetic biology, and the development of serum-free culture media. Public acceptance remains a hurdle, with concerns over scalability, cost, and safety. |
| 4 | Quality and Risk Control in Cultivated Meat Production Check for updates | de Macedo et al. (2024) | Quality control and risk assessment in cultivated meat production | Emphasized the importance of reproducibility, donor selection, and cell culture safety in cultivated meat production. Identified the need for strict microbiological controls, environmental monitoring, and addressing risks like heavy metals, toxins, and pathogens. Highlighted the significance of regulatory frameworks to ensure consumer trust and safety in the final product. |
| 5 | Biotechnological and technical challenges related to cultured meat production | Lanzoni et al. (2022) | Challenges and approaches for large-scale cultured meat production | Identified key steps and challenges in cultured meat production, such as animal selection, FBS alternatives, and scalable biofabrication systems. Biotechnological hurdles include replicating traditional meat’s nutritional and functional quality, while technological challenges focus on optimizing scaffolding, 3D bioprinting, and bioreactors for large-scale production. |
| 6 | Considerations for the development of cost-effective cell culture media for cultivated meat production | O’Neill et al. (2021) | Challenges in designing cost-effective culture media for large-scale production | This study focuses on the critical role of culture media in cultivated meat production. It highlights the need for developing affordable, food-grade, and animal-ingredient-free media to support large-scale muscle cell proliferation and differentiation. Drawing insights from conventional culture media applications and microbial fermentation processes, it emphasizes that overcoming media-related challenges will be crucial for successful commercialization of cultivated meat. |
To avoid this issue, researchers have explored the use of nutrients derived from plants and microalgae as a sustainable and environmentally friendly alternative to conventional medium components (Okamoto et al., 2020). Moreover, to avoid the use of animal-derived serum, which poses risks of contamination and ethical concerns, serum-free media are being developed and optimized (Messmer et al., 2022).
To ensure the safety and high quality of CBM products, the precise regulation of cell proliferation and differentiation into specific tissues, such as muscle and fat, is vital to prevent the formation of undesired cell types or structures (Fish et al., 2020; O’Neill et al., 2021). Myogenesis, the process of muscle stem cell development and growth in vitro, constitutes a pivotal stage in CBM production. This complicated process commences with the collection of muscle samples for stem cell isolation, followed by tissue separation, primary cell culture, scaled-up cultivation, muscle differentiation, maturation, and tissue extraction (Kadim et al., 2015). Despite advancements in muscle stem cell research, optimizing these procedures remains challenging, thereby hindering the efficient production of meat derived from muscle cells in vitro (Choi et al., 2021).
Efficient myogenesis involves not only the proliferation and differentiation of muscle stem cells but also the precise regulation of various detrimental factors, including growth factors, cytokines, and substrate stiffness, to replicate the natural cellular process accurately (Rafi et al., 2021). Moreover, maintaining the purity and stability of differentiated muscle cells throughout the cultivation process is crucial for ensuring product consistency and adhering to safety standards (de Macedo et al., 2024). Overcoming these challenges is essential for scaling up production and satisfying the stringent safety and quality criteria required for lab-grown meat to become a viable alternative to conventional meat production methods (Guan et al., 2022).
Cells maintained in continuous culture are prone to accumulating genetic mutations over time, primarily due to replication errors, environmental stress, and the aging of cell lines (Martins et al., 2024). In the course of DNA replication, intermittent errors, such as base mispairings, insertions, or deletions, may occur despite the presence of repair mechanisms, resulting in permanent alterations to the genetic sequence (Ray, 2022). Additionally, the culture environment, which frequently diverges from the natural conditions that support cell growth, may expose cells to various stressors, including nutrient depletion, and oxidative stress (Lee et al., 2016; Martincorena and Campbell, 2015). As cell lines age, their ability to repair DNA effectively declines, which accelerates the accumulation of mutations. Furthermore, selective pressures in the culture environment may favor cells that acquire beneficial mutations, resulting in genetic drift and increased heterogeneity within the cell population. (Chandrababu and Puthumana, 2024; Zhang et al., 2020). In the context of CBM, the accumulation of mutations poses a significant challenge, as these transformations can lead to the loss of essential cellular functions, a reduction in the nutritional value of the final product, and potentially oncogenic alterations that promote uncontrolled cell proliferation (Hauser et al., 2024).
Regular genetic monitoring is requisite to maintain the genetic stability and intended characteristics of cultured cells. This involves conducting genetic and functional testing to determine the maximum number of cell passages permissible in the laboratory without exhibiting significant changes or loss of function (Jaime-Rodríguez et al., 2023). For instance, exome and whole-genome sequencing are robust techniques for obtaining comprehensive molecular profiles of genetic alterations. Additionally, RNA sequencing provides insights into gene expression changes. In contrast, epigenomic approaches such as DNA methylation profiling and chromatin immunoprecipitation sequencing reveal modifications that regulate gene activity (Kuraz Abebe et al., 2024). Proteomics and metabolomics enhance this understanding by providing insights into protein expression and metabolic changes, thereby suggesting a comprehensive interpretation of molecular alterations at the genetic, transcriptional, and biochemical levels (Sandhu et al., 2023).
Chemical Safety of Cell-Based Meat
The production of lab-grown meat, like other cell culture processes, encounters significant challenges related to potential microbial contamination from environmental sources, equipment, and/or personnel. CBM production begins with the extraction of stem cells or myoblasts from animals. Contamination of these cell lines with bacteria, fungi, or viruses at this initial stage can compromise the entire production batch of the cell culture production process (Van der Gucht, 2018). For example, the rapid proliferation of bacterial contaminants, such as Escherichia coli, and fungal pathogens, like Mycoplasma hyorhinis, can compromise both the safety and quality of the CBM production process (Xiong et al., 2016). Moreover, growth medium, often containing nutrients, growth factors, and animal-derived serum (like fetal bovine serum, or alternatives), can be a significant source of microbial contamination (Butler, 2015). Similarly, upon harvesting CBM, poses contamination risks during processing, packaging, and storage. Inadequate hygiene, improper handling, and contact with contaminated surfaces or equipment can introduce pathogens into the final product (Sogore et al., 2024).
Additionally, in CBM production, bacterial and fungal contamination poses persistent challenges that are frequently managed using antibiotics. However, this approach is marred by several drawbacks, including incomplete microbial eradication, limited antibacterial efficacy, and the risk of recontamination (Shi et al., 2019). This highlights the dangers associated with antibiotic use in CBM production (Qamar et al., 2023). The emergence of antibiotic resistance in bacteria found in meat products, especially against antibiotics such as tetracycline, penicillin, and methicillin, has extensively been documented (Abbasi et al., 2021; Qamar et al., 2023).
Scaffolds play a vital role in facilitating cell growth and tissue formation during CBM manufacture. Scaffolds must be generated from biocompatible and non-toxic materials to ensure the preservation of cell viability and the safety of the final product (Seah et al., 2022). Biodegradable polymers, such as polylactic acid and polycaprolactone, are frequently utilized as scaffold materials. These materials are preferred owing to their non-toxic nature and ability to decompose harmless byproducts within the body. Furthermore, scaffolds can utilize crosslinking agents to strengthen their mechanical characteristics. A thorough evaluation is necessary to ensure that these compounds and any residues do not remain in the final product (Bomkamp et al., 2022; Seah et al., 2022).
In addition to synthetic polymers and crosslinking agents, the use of natural, plant-based materials as scaffolds in the synthesis of CBM is gaining interest. Materials such as alginate, which is derived from seaweed, as well as gelatin and cellulose, are currently being investigated for their biocompatibility and functional characteristics. Plant-based scaffolds obtained from natural sources possess the benefit of being renewable and can be designed to degrade at certain rates that are optimal for tissue development (Wang et al., 2023). The safety of these natural materials is determined via thorough examination, which entails testing for potential allergens, toxins, and microbial contamination. Ensuring that these plant-derived scaffolds do not introduce any hazardous compounds into the cell culture or end product is requisite to preserving the integrity and safety of CBM. Safety evaluations for these materials involve rigorous testing for potential toxicity, immunogenicity, and long-term biocompatibility to ensure they fulfill the stringent standards required for food safety and consumer health (Lee and Choi, 2024).
In the CBM production system, the bioreactor is an integral component, designed as a specialized, closed system that creates a controlled environment mimicking the conditions inside a living organism (Kendall, 2022). This environment provides the optimal conditions for animal cells to grow, proliferate, and differentiate into muscle tissue, which forms the basis of CBM. Through regulating factors such as temperature, pH, oxygen levels, and nutrient supply, bioreactors enable the cells to develop into structured tissues, ensuring efficient and consistent production of CBM (Azhar et al., 2023). Therefore, bioreactors must be designed and operated under sterile conditions to prevent contamination from bacteria, fungi, or viruses. Sterilization techniques such as steam-in-place and clean-in-place are crucial to maintaining aseptic conditions (Dutta et al., 2024). Moreover, air filtration systems using HEPA (High-Efficiency Particulate Air) filters, combined with automated systems that reduce human intervention, are vital for minimizing the risk of contamination in bioreactors. As cells are cultured, the expansion of bioreactor designs must address both the biological demands of cell growth and the engineering challenges associated with large-scale operations, ensuring that conditions remain sterile and conducive to optimal cell development (Allan et al., 2019; Negulescu et al., 2023).
Regular monitoring of microbial contamination at all production stages is necessary to promptly detect and address issues. Continuous monitoring systems integrated into bioreactor designs ensure rapid detection and control of any contamination. In this context, modern sensors and control systems serve an important role in providing real-time data on the bioreactor environment. For instance, using disposable sensors and advanced control systems in bioreactors with rocking motion has reduced the possibility of contamination while increasing overall production efficiency (Glazyrina et al., 2010). Furthermore, implementing fed-batch control strategies and internal substrate delivery systems can help maintain optimal conditions for cell growth and minimize the likelihood of contamination (Zhang et al., 2020).
Nutritional Quality and Safety of Cell-Based Meat
Ensuring that the final CBM product undergoes rigorous testing for residues from growth media, antibiotics, and/or other chemicals used during production is crucial for consumer safety and the maintenance of public health standards. A study on antibiotic residues in raw meat revealed that a considerable proportion of samples contained residues of ciprofloxacin, streptomycin, tetracycline, and sulfanilamide, with certain concentrations exceeding the recommended limits (Ramatla et al., 2017). These findings underscore the importance of stringent testing protocols in CBM production to avoid similar challenges.
Furthermore, food additives can be employed in CBM manufacture to improve flavor, texture, and shelf life. To protect consumer safety, regulatory organizations must classify these compounds as “Generally Recognized as Safe (GRAS).” This grade implies that experts consider the additive safe for ingestion, as corroborated by robust scientific facts. Additives should be identified by their E-numbers or chemical names to enable informed decision-making. Comprehensive testing and continuous monitoring are essential for maintaining the rigorous safety standards required for CBM products (Fraeye et al., 2020). However, challenges persist in regulating the protein, fat, and micronutrient content of lab-grown meat. Technological advances, such as three-dimensional printing, are being explored to resolve these challenges, offering potential solutions for optimizing the nutritional profile of lab-grown meat (Handral et al., 2022).
Regulatory frameworks for CBM vary significantly across countries, reflecting diverse sociopolitical contexts and governing ideologies. According to a report by the Good Food Institute, under a formal agreement established in 2019, the US Food and Drug Administration (FDA) and the US Department of Agriculture’s Food Safety and Inspection Service (USDA-FSIS) share regulatory oversight of cultivated meat. The FDA is responsible for cell collection, banking, and cultivation across all species, while the USDA-FSIS oversees the processing, packaging, and labeling of cultivated meat, poultry, and catfish products. Additionally, the FDA retains jurisdiction over the processing, packaging, and labeling of other cultivated seafood and game meat products (Diaz, 2023; Lee and Choi, 2024).
Furthermore, a comprehensive report on the regulatory aspects of CBM was recently published (Diaz, 2023). The author provides a detailed description of how the European Food Safety Authority (EFSA) regulates lab-grown meat in the EU, conducting a thorough safety assessment before its commercialization. This includes assessing potential concerns such as microbiological safety, chemical hazards, and allergenicity, as well as comparing the nutritional profile to traditional meat. EFSA inspects the entire manufacturing process, from cell procurement to finished product, to ensure safety and the absence of hazardous contaminants (European Food Safety Authority et al., 2024). This regulatory framework extends to CBM, where the EU’s precautionary principle and diverse member state policies complexify market entry and commercialization. The political and institutional ambiguities within the EU further complicate the establishment of a cohesive regulatory system.
In Asia, the regulatory landscape for CBM is evolving, with countries such as Singapore adopting a proactive approach. Singapore has emerged as a global pioneer by becoming the first country to approve the sale of CBM, demonstrating a progressive commitment to food innovation and safety. Other Asian countries are gradually developing their regulatory frameworks, often influenced by North American and European standards (Smyth and Phillips, 2014). For instance, in Korea, the Ministry of Food and Drug Safety (MFDS) regulates new food ingredients through the New Food Raw Material Recognition System, which permits the temporary use of novel ingredients following a comprehensive safety review. This system, similar to the GRAS framework in the US and the Novel Food regulation in the European Union, encompasses agricultural, livestock, and marine products, as well as microorganisms. To date, 54 items, including edible insects as alternative protein sources, have been approved under this system (Lee et al., 2022; Lee et al., 2024). Table 2 summarizes the published literature regarding the safety and regulatory aspects of CBM.
| No. | Title | Authors | Country | Safety concerns | Regulatory aspects | Key findings |
|---|---|---|---|---|---|---|
| 1 | The barriers and drivers of a safe market introduction of cultured meat: A qualitative study | Ketelings et al. (2021) | The Netherlands | The lack of sufficient research on hazard and risk characterization constitutes a significant barrier. It is recommended to conduct genetic stability assays for starter cell lines and to perform residual testing for culture medium and serum. | Compliance with EFSA guidelines under Novel Food or GMO regulations is required. Global regulatory inconsistencies need addressing for smoother market entry. | Certain areas of cultured meat research require more attention from researchers to ensure the highest level of safety. Overall, the lack of in-depth research related to hazard and risk characterization of cultured meat is considered the biggest barrier in introducing a safe product to the market. |
| 2 | Scientific, sustainability and regulatory challenges of cultured meat | Post et al. (2020) | The Netherlands | Essential to use serum-free medium and optimize biomaterials and genetic stability assays for starter cell lines and residual testing for culture medium and serum, are key safety concerns. Additionally, ensuring high-volume cell production in industrial bioreactors using a serum- free medium and establishing clear regulatory pathways are essential for the safe commercialization of cultured meat. | FDA and USDA–FSIS: FDA oversees early cell development; USDA–FSIS manages final production and labeling. Agencies collaborate but have separate roles. Regulations: Cultured meat is regulated under Novel Foods or GMO legislation, with a framework in place since 1997 and updated in 2018. | Key safety findings for cultured meat include insufficient research on hazard and risk characterization, necessitating genetic stability assays and residual testing for culture medium and serum. Achieving scalability and cost efficiency is crucial, with high-volume cell production in serum-free media being essential for safe manufacturing. Clear regulatory pathways are also necessary for safe commercialization. |
| 3 | The business of cultured meat | Choudhury et al. (2020) | Singapore | Growth media: Must contain necessary nutrients for cell proliferation. Challenges include optimizing media and ensuring it supports scalable production. | - Lack of framework: No established regulatory framework specifically for cultured meat. - Existing regulations: Safety is recognized but CM lacks clear regulatory guidelines. - Consumer perception: Concerns include taste, texture, and misleading labels. |
- Commercialization challenges: These include optimizing growth media, improving taste and texture, and overcoming consumer biases. - Geographical spread: Culture meat companies are globally distributed, with significant investments and growing interest in North America, Asia, and Europe. - Funding: Significant investments from both public and private sectors are crucial for advancing culture meat production. |
| 4 | Food safety considerations and research priorities for the cultured meat and seafood industry | Ong et al. (2021) | USA | - Hazard identification: Focuses on identifying potential chemical and biological hazards in the manufacturing process of cell-cultured meat and seafood. - Assessment methods: Existing safety assessment methods from conventional foods, biotechnology, and pharmaceuticals are applicable, but additional evaluation of novel inputs may be necessary. |
- Framework development: No existing regulatory framework specifically for cell-cultured meat and seafood; however, established principles from related fields can inform safety frameworks. - Harmonization: Existing global standards for quality management and safety testing may apply, but gaps in knowledge and novel contaminants require further attention. - Standardization: Development of standardized lists for residues, byproducts, and contaminants will enhance product testing efficiency. |
- Modular manufacturing process: A generalized modular diagram was created to identify hazards across different manufacturing processes and enable tailored risk management. - Consumer acceptance: A data-driven, transparent approach to safety can support consumer acceptance and realization of cell-cultured products’ potential. |
| 5 | Safety of alternative proteins: Technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein | Hadi and Brightwell (2021) | New Zealand | - Cultured meat: Risks from viruses, prions, and genetic engineering. Serum-based media may pose hazards. - Plant-Based Meat: Risks include allergens, anti-nutrients, and potential carcinogens. - Insect protein: Concerns about microbiological safety and allergens. - Single-cell protein: Risks include toxins, allergens, and high RNA content. |
- Cultured meat: Regulated as novel food; overseen by FDA and USDA-FSIS in the USA, and by European regulations in Europe. - Plant-based meat: Regulated similarly to non-animal foods; certain components may need novel food approval. - Insect protein: Governed by novel food regulations; recent approvals in Europe. - Single-cell protein: May need GRAS status in the USA; subject to novel food regulations in Europe. |
- Cultured meat: Requires more research on contaminants and health effects. - Plant-based meat: Needs further study on health impacts and processing risks. - Insect protein: More research needed on allergens and microbiological safety. - Single-cell protein: Focus on toxin and allergen risks. - Global standards: Need for comprehensive global safety regulations. |
| 6 | Technological, regulatory, and ethical aspects of in vitro meat: A future slaughter‐free harvest | Bhat et al. (2019) | New Zealand | - Chemical and microbial safety: Cultured in a sterile bioreactor, reducing pathogens and chemical hazards. - Potential risks: Novel materials used may pose untested risks; genetic instability and contamination of cell lines or media are concerns. - Health risks: No living cells in final product; recombinant proteins should not pose novel risks. |
- Current status: Limited to research; no established commercial regulations yet. - Oversight: Likely to involve food safety authorities; in the US, collaboration between USDA and FDA is anticipated. - Special considerations: Regulation will need to address unique aspects like culture media and scaffolds. |
- Sustainability challenges: Environmental impacts and cost of large-scale production need addressing. Sustainability of cultured meat systems remains uncertain. - Technological barriers: Development of animal-free culture media and efficient bioreactors is crucial. Current reliance on fetal calf serum and animal-derived scaffolds is unsustainable. - Economic and social factors: Cost, social acceptance, and scalability are central challenges. Effective product-oriented publicity may drive consumer adoption. |
| 7 | US lawmakers float plan to regulate cultured meat | Servick (2018) | USA | - Safety assessment: Unclear processes for assessing safety; current regulations focus on traditional meat production, which differs significantly from cultured meat methods. - Concerns: Debate over whether USDA has the necessary expertise; potential for new risks related to the novel production process. |
US regulation: - Proposed oversight: USDA would oversee cultured meat manufacturing and labeling as per recent House bill language. - Industry disputes: Some argue that the USDA’s approach may lead to unnecessary regulations; concerns about lack of expert input. - Possible alternatives: FDA may also play a role, given its expertise in cell and tissue-based products. |
- Technological impact: Cultured meat technology promises reduced animal suffering, lower energy and land use, and fewer greenhouse gas emissions. - Regulatory debate: Ongoing discussions about appropriate regulatory bodies and standards. USDA’s ability to regulate cultured meat is questioned due to its traditional focus on livestock. - Global perspective: Different countries, including the EU, are developing their own regulations for cultured meat, reflecting varying approaches to safety and market introduction. |
| 8 | Cultured meat safety research priorities: Regulatory and governmental perspectives | Ong et al. (2023) | USA | - Need for new analytical methods or adaptation of existing ones for diverse cultured meat and seafood products. - Testing for residues of media ingredients, structural materials, cells, and bioactive molecules. - Common hazard prevention methods (e.g., HACCP, GMP) are deemed sufficient once contamination sources are understood. - Monitoring for pathogens and chemical compounds from both traditional and novel production environments. |
- Safety and regulatory assessments should account for novel production processes and potential new hazards. - Existing approaches can be adapted for culture meat products, but specific criteria and methods need to be developed. - Emphasis on creating databases and sharing data from both private and public sectors to support safety evaluations and regulatory framework development. - Transparency in risk assessment is crucial for building consumer trust. |
- Culture meat products may differ significantly from conventional products, requiring tailored safety evaluation criteria. - Compositional and process differences necessitate novel parameters for safety assessments. Unique production processes introduce potential new hazards, such as genetic and metabolic stability issues. No universal safety standards are currently available; products need to be assessed individually until standardized methods emerge. |
| 9 | Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture | Stephens et al. (2018) | UK | - Cell source and culture media: Challenges in replicating the in-vivo muscle growth environment and finding effective culture media. - Bioprocessing: Need for bioprocessing methods that can scale to commercial production while ensuring product affordability. - Contaminants: Lower purity of raw materials is acceptable compared to biomedical applications, but ongoing monitoring needed for contaminants and residues. |
- Production: Regulatory frameworks need to address the novel technical aspects of cultured meat production, including scalability and cost-efficiency. - Public acceptance: While consumer acceptance is crucial, the broader political and institutional framework also affects regulation and industry development. - Regulatory frameworks: Need for comprehensive regulations that address both technical and socio- political aspects of cultured meat production. |
- Technical challenges: Large-scale production and affordability are difficult; significant climate impacts may take decades. - Social and institutional issues: Beyond consumer acceptance, socio-political factors influence development; economic instability and start-up failures are risks. - Environmental benefits: Cultured meat may not inherently deliver all benefits; should be part of a broader strategy including meat reduction and policy reforms. |
| 10 | Risk assessment of cultured meat | Gu et al. (2023) | China | - Hazards and risks: Hazards and risks may be introduced at any stage during the production of cultured meat. - Risk assessment: Effective risk assessment strategies are necessary to ensure the safety of cultured meat. - Standardized practices: Implementation of good laboratory practices, good manufacturing practices, good cell culture practices, and codes of hygienic practices is essential for safe production. |
- Novel food status: Cultured meat is considered a novel food, requiring evaluation by regulations or legislation of respective jurisdictions before market introduction. - Harmonized framework: The regulatory framework for cultured meat may differ across regions but should eventually be harmonized to promote safe and nutritious cultured meat products globally. |
- Sustainability potential: Cultured meat has the potential to become a sustainable source of nutritional protein. - Technical challenges: There are significant technical challenges in producing cultured meat in large quantities. - Lack of transparency: Production processes remain largely undisclosed due to trade secrets, impacting transparency and safety assurance. - Ethical and health benefits: Cultured meat is perceived to have relative ethical and health advantages over conventional meat. |
| 11 | Cultured meat and challenges ahead: A review on nutritional, technofunctional and sensorial properties, safety and legislation | Broucke et al. (2023) | Belgium | - Food safety risks: Cultured meat production involves potential risks such as microbial contamination, prions, and genetically engineered starting materials. - Controlled production environment: The enclosed and controlled environment of in vitro meat production is believed to reduce the risk of animal diseases, foodborne illnesses, antibiotic-resistant pathogen strains, and exposure to chemical hazards. |
- Cultured meat lies at the boundary between meat and non-meat, necessitating clear definitions and appropriate regulatory frameworks. Current discussions focus on EU and US regulations. | - Nutritional profile: Cultured meat consists of in vitro cultivated animal cells, producing proteins, fatty acids, growth factors, and cytokines. - Technofunctional and sensorial properties: There are significant differences between cultured meat and traditional meat in terms of texture, color, flavor, and overall sensory experience. - Processing impact: The impact of further processing on the quality of cultured meat, including protein quality and sensory properties, is still a subject of scientific research. - Production challenges: Cultured meat production faces challenges such as scaling up, optimizing bioreactors, and developing bioprinting techniques for producing complex multicellular tissues. |
| 12 | Cell-based meat labeling–Current worldwide legislation status: A review | Vlčko et al. (2023) | Poland | - Safety evaluation: In the EU and other regions, cell-based meat products are expected to undergo a safety evaluation under novel food legislation, though no applications have been registered yet. - Risk of stigmatization: Legal frameworks in some US states might impede market introduction or cause stigmatization of cell based meat products, impacting consumer perception. |
- Global approval status: Singapore is the only country that has approved cell-based meat for market placement. - US regulatory framework: The US has established a regulatory framework where the USDA and FDA will control cell-based meat matters. - EU Novel Food Regulation: Cell-based meat products in the EU will be evaluated under the Novel Food Regulation, with additional guidelines for food business operators. - Other countries: Countries like Canada, Australia, New Zealand, Japan, and Israel are expected to evaluate cell-based meat under their novel food legislation. - Labeling regulations: There is a lack of clear legislation on the labeling of cell-based meat products in most countries. |
- Increasing investment: There is growing investment in cell-based meat technology by major food industry corporations. - Anticipated market launch: Many companies are announcing plans to launch cell based meat production in several markets worldwide in the coming years. - Policymaker considerations: Policymakers should avoid implementing local laws that could negatively impact consumer perception of cell-based meat technology while ensuring clear labeling to distinguish product origin. - Implementation timeline: The introduction of cell-based meat to many markets is expected to take months or years, not weeks, due to the time required for regulatory approval and safety evaluations. |
The regulatory framework for CBM, particularly regarding its pre-market approval processes, is characterized by complexity and diversity, reflecting the early stage of this transformative technology. The pre-market approval process for CBM requires several critical steps, including comprehensive safety assessments, nutritional evaluations, and adherence to established food safety standards. Regulatory bodies, such as the FDA in the US and the EFSA, are actively developing robust frameworks designed to ensure the safety of these products for human consumption (FDA, 2023). Central to this process is a thorough analysis of the cell lines employed, the specific growth conditions, and the bioprocessing techniques utilized in the development of lab-grown meat. The FDA has implemented a pre-market consultation process for cultivated meat, requiring each company to submit a range of data and information that clearly demonstrates how and why the product is safe for human consumption. During this consultation, the FDA reviews and evaluates the information provided, assessing the company’s entire production process, including the establishment of cell lines and cell banks, the proliferation and differentiation of cells, the cultivated cell material, and all components and inputs involved in manufacturing controls. The FDA may also request additional information and data as needed. Once the agency is satisfied that it has all the necessary information and completes its evaluation, it informs the company that it has no further questions or concerns (Diaz, 2023; Vlčko et al., 2023).
Additionally, the regulatory approval procedure should consider the potential risks associated with the use of synthetic and animal-derived substances in culture media. Safeguarding the final product from detrimental toxins, allergens, pathogens, and other hazardous elements introduced during production is of utmost importance (Stephens et al., 2018). Recent studies have suggested that optimizing culture media through plant-based alternatives may mitigate some risks associated with animal-derived components while enhancing the nutritional profile of CBM (O’Neill et al., 2021; Rubio et al., 2020; Wali et al., 2024).
The regulatory frameworks governing CBM, particularly concerning labeling and consumer information, are characterized by complexity and rapid evolution. A significant challenge in obtaining regulatory approval for lab-grown meat is the lack of a well-defined legal framework specifically applicable to this innovative product. Traditional meat products are regulated under several established acts, such as the “Livestock Industry Act,” “Food Sanitation Act,” and “Livestock Products Sanitary Control Act.” However, CBM does not fit neatly into these categories, as it is produced without conventional livestock breeding. As a result, there is currently no clear legal framework to guide the application of existing standards and requirements to CBM (Ketelings et al., 2021). Efficient methods of communication are requisite to educating consumers regarding the advantages and safety of the final product. The public’s perspective is shaped by multiple elements, such as ethical issues, nutritional content, and familiarity with the product. Transparent and informative labeling can boost customer trust and adoption by addressing concerns regarding the naturalness, safety, and nutritional profile of CBM (Kouarfaté and Durif, 2023).
Consumer education initiatives should accompany labeling efforts for CBM to clarify its production process and benefits for animal welfare and sustainability. Engaging consumers in discussions about scientific advancements can alleviate concerns and promote informed decisions. Incorporating feedback mechanisms, such as surveys, allows manufacturers and regulators to understand consumer expectations (Tai, 2019). Regulatory agencies should consider third-party certifications to enhance confidence in safety and sustainability. Additionally, using clear, concise language on labels and incorporating visual aids can improve consumer understanding of the health benefits and environmental impacts of CBM (Bryant, 2020).
Post-market surveillance for CBM requires ongoing monitoring to ensure that the products adhere to safety and quality standards. Preventing concerns such as misrepresentation of food and adulteration is indispensable. CBM regulatory authorities must establish and enforce sensor-based technology measures to address fraud and unintentional mislabeling. This encompasses the utilization of predictive microbiological models, such as the Temperature Function Integration (TFI) model, which has proven effective in traditional meat hygiene regulatory practices. The TFI model enables regulators to measure and control possible microbial growth in meat products, guaranteeing both hygiene and commercial efficiency (Armitage, 1997). Moreover, effective monitoring and reporting mechanisms are critical to maintaining the transparency and traceability of CBM products. Regulatory authorities must develop frameworks that require precise reporting on production processes, such as the cell source, culture medium composition, and bioprocessing technologies. Transparency is requisite to gaining consumer trust and guaranteeing product safety. An interdisciplinary approach to CBM production, which includes continual laboratory research and expert consultations, emphasizes the necessity of a comprehensive regulatory framework that tackles both technical and ethical aspects (Djisalov et al., 2021; Stephens et al., 2018).
Conclusions and Future Directions
CBM represents a promising alternative to conventional meat, with potential benefits for animal welfare and natural resource conservation. However, significant challenges persist in ensuring its biological and chemical safety, nutritional quality, and regulatory compliance. Biological safety is crucial, starting with careful sourcing of animal cells and conducting aseptic biopsies to prevent contamination. Using non-invasive techniques can enhance animal welfare and uphold ethical standards while understanding muscle cell growth is necessary to ensure proper differentiation. Chemical safety addresses the challenge of microbial contamination, which can arise from environmental sources, equipment, and personnel. It is essential to reduce reliance on antibiotics and maintain sterile conditions. Additionally, choosing biocompatible and biodegradable materials helps prevent harmful residues. Ensuring the nutritional quality and safety of CBM involves rigorous testing for harmful residues and thorough evaluation of food additives. The regulatory framework for lab-grown meat varies worldwide. In the US, oversight is shared between the FDA and USDA-FSIS, while the EU emphasizes comprehensive safety assessments. Singapore’s proactive approach serves as a model for commercialization, whereas the MFDS regulates new food ingredients in Korea. However, many regions still lack cohesive regulatory frameworks to promote the acceptance of CBM. In summary, successfully commercializing CBM depends on strategies addressing biological and chemical safety, nutritional integrity, and regulatory compliance. Ongoing research and collaboration among stakeholders will be vital to overcoming challenges and realizing CBM's potential as a sustainable and ethical alternative to traditional meat production.