Introduction
Dairy milk is consumed daily as food. In 2022, the global value of the dairy market was estimated at 893 billion USD and is projected to grow to 1,243 billion USD by 2028 (Shahbandeh, 2023). This commodity comes in various forms including fresh, powdered, and synthesized products. As projected, the last few years have witnessed rapid global cow milk production with China, Pakistan, the US, the EU, and India at the forefront (Harvey and Dijs-Duijndam, 2023). This expansion is also associated with an increased generation of dairy waste, which can be broadly divided into effluent (wastewater) and solid (sludge) waste (Ashekuzzaman et al., 2020). The wastes are created at various stages including production, processing, packaging, and shipping of the finished goods (Ashekuzzaman et al., 2020). Dairy wastewater (DWW) typically exhibits high chemical oxygen demand (COD) and biological oxygen demand (BOD), with values ranging from 1 to 10 g/L for COD and 0.3–5.9 g/L for BOD (Kothari et al., 2017). When wastewater is discharged into water bodies, the organic content depletes dissolved oxygen, hurting aquatic lives and the environment. Fatty and oily fat effluents create a surface layer on water bodies that prevents oxygen from getting to aquatic plants and animals (Adetunji and Olaniran, 2021). The high organic load of dairy industry waste necessitates proper treatment before disposal. However, treating and disposing of such waste are challenging due to the substantial costs and complex processes involved. In this context, the paradigm shift from perceiving waste as a burden to viewing it as a potential resource becomes imperative. Waste valorization is a pivotal solution in contemporary sustainability paradigms, aiming to repurpose waste materials into valuable commodities, fostering a circular economy while mitigating environmental degradation (Adesra et al., 2021).
Research endeavours in waste valorization underscore the significance of resource recovery, aligning with broader goals of social and environmental sustainability (Adesra et al., 2021; Capanoglu et al., 2022). However, challenges persist, particularly concerning the management of sludge generated during waste treatment processes, necessitating innovative technological interventions to extract value from waste streams while ensuring sustainable waste management practices.
The environmental consequences of dairy waste can be detrimental, as it also contributes to the emission of greenhouse gases (GHGs), which in turn exacerbate global warming. Therefore, effective waste management or valorization strategies are necessary to reduce these impacts. Transforming dairy waste into valuable resources through biological or thermochemical conversion pathways is one of the ways to address this issue of dairy waste (Okolie et al., 2022; Walsh et al., 2022). Thermochemical techniques like gasification, pyrolysis, and liquefaction utilize heat and chemical energy at high temperatures (Ramola et al., 2020). On the other hand, biological methods such as anaerobic digestion and fermentation use microorganisms to break down dairy waste. Utilizing these waste valorization techniques can advance a safe environment.
There are a handful of review articles on the valorization of dairy wastes. Usmani et al. (2022) published a review of the valorization of dairy waste through microbes as a sustainable and green method to generate biofertilizers, biofuels, power, and other biobased products. Ozcelik et al. (2024) focused on the valorization of dairy side streams using microalgae to provide an understanding of the issues and challenges of valorizing industrial dairy side streams as substrates for value-added microalgal biomass production. Chaudhary et al. (2023) also recently reviewed the potential of microorganisms in converting dairy wastes into value-added products. Their emphasis was on recent developments in valorization technologies of dairy effluents and other by-products using different microbial cell factories. There is also a report on the valorization of dairy wastes through the integrative approaches for value-added products (Adesra et al., 2021). Although these reports are insightful, they only focus on a specific technique. This current work therefore intends to harness and give a comprehensive overview of the various valorization methods that have been used to convert dairy wastes into value-added products. The processes covered include thermochemical [hydro-thermal carbonization (HTC), hydro-thermal liquefaction (HTL), pyrolysis, and gasification], biological (anaerobic and aerobic), and integrated approaches. The work also identifies research gaps and professes future research direction for the conversion of dairy wastes to value-added products.
Types, Sources, and Characteristics of Dairy Wastes
Dairy waste is generally divided into two main categories: solid waste, also known as sludge, and wastewater, referred to as effluent (Fig. 1A; Kwapinska et al., 2020). Sludge or solid waste can be further categorized into chemical and biological sludges. The sludge resulting from dairy waste treatment contains both degradable organic matter and non-biodegradable solid matter, and its volume is directly linked to the amount of wastewater produced. For instance, a dairy facility processing around 500,000 liters of milk daily can generate between 200 to 350 kg of sludge. The management and disposal of this sludge can be a major challenge, accounting for 60% of the overall operating cost of a treatment plant (Jayashree et al., 2014). Conversely, wastewater or effluents possess an excessive organic load owing to the occurrence of carbohydrates, proteins, and fats originating from milk (Kushwaha et al., 2011). The high organic content in DWW necessitates proper treatment, as it can cause rapid oxygen depletion upon discharge. For every liter of milk produced, the dairy industry generates 1–3 liters of wastewater (Jayashree et al., 2014; Settanni et al., 2020).
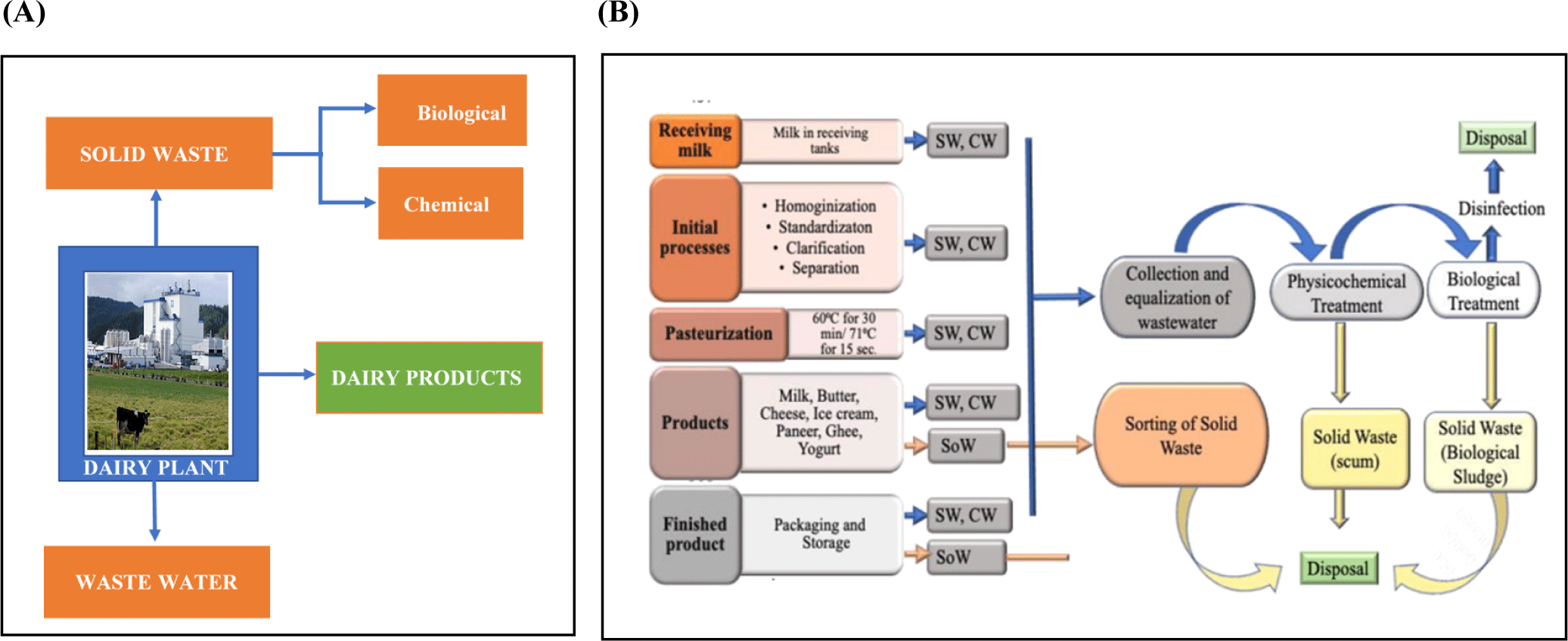
The sources of dairy waste stem from the various processes ranging from the point of reception to transportation, manufacturing, and packaging of products. Fig. 1B illustrates the different processes of dairy waste generation in milk processing. In some cases, an attempt to maintain sanity in milk processing units results in waste generation that contains cleaning agents such as detergents. Excessive product leakage, overflow, and inadequate control measures also contribute to waste production in dairy processing. As a result, the sludge generated contains waste from various sources, including processing, cleaning operations, and physicochemical and biological treatment processes (Usmani et al., 2022).
The characteristics of dairy waste are more often determined by studying the physicochemical properties of the wastewater. Generally, DWW possesses high organic contents and a wide pH range. As previously mentioned, the discharge of untreated DWW into water bodies results in water quality deterioration and ecological imbalance. The volume and features of wastewater produced by a dairy factory may vary in terms of composition, concentration, and quantity. These parameters depend on constituents such as the type of product processed, operational methods, plant design, wastewater treatment utilized, and water consumption (Wang et al., 2005). Seasonal changes also influence the composition of wastewater. Table 1 summarizes the physicochemical properties of DWW reported in the literature and the discharge standards from various bodies. For sludge, its typical characteristics are determined by the treatment process employed and can differ based on the treatment type and chemicals used during the physicochemical process (Ashekuzzaman et al., 2019).
| S/N | pH | TDS (ppt) | BOD (mg/L) | COD (mg/L) | SS (mg/L) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 7.4 | - | 1,710 | 2,520 | 1,020 | Passeggi et al. (2009) |
| 2 | 6±0.69 | 1.28±0.25 | 355±78.99 | 982±67.57 | - | Wang et al. (2005) |
| 3 | 6.8±0.64 | 1.2±0.25 | 320±26.76 | 954±86.18 | - | Wang et al. (2005) |
| 4 | 7–8 | 1.300 | 1,040 | 2,100 | 1,200 | Khan (2017) |
| 5 | 6.4 | 2.180 | 1,445±30 | 4,410±60 | 1,660±60 | Suman et al. (2017) |
| 6 | 4–12 | 300–1,400 | 650–3,000 | 250–2,700 | Cecconet et al. (2018) | |
| 7 | 9.8 | 1.222 | 650 | 1448 | - | Ashekuzzaman et al. (2019) |
| Arab | 6.7–9.1 | - | 1,941±864 | 3,383±1,345 | - | Flayyih and Ali (2022) |
| Central Pollution Control Board | 6.5–8.5 | - | 100 | - | 150 | |
| World Bank | 6–9 | - | 50 | 250 | 10 | Flayyih and Ali (2022) |
| Turkey | 6–9 | - | - | 160 | 30 | Flayyih and Ali (2022) |
| Iraq | 6–9.5 | - | <40 | <45 | 60 | Flayyih and Ali (2022) |
Dairy Wastes Treatment Technologies
The prevalent disposal approaches in several countries are landfilling and incineration (Assamoi and Lawryshyn, 2012). These techniques, despite their widespread use, are inherently unsustainable. The main issues with these techniques are their negative impact on the environment and resource conservation. The increased waste generation occasioned by the rapid population growth has rendered these traditional approaches unsatisfactory. Waste valorization has helped achieve a circular economy and environmental sustainability (Audu et al., 2020). Waste valorization technologies can be categorized into thermochemical and biological processes. Both technologies can be integrated to achieve maximum performance (Parashar et al., 2016). These valorization technologies are also useful in converting dairy wastes to value-added products. The by-products of dairy waste valorization are presented in the section titled ‘Processes and Mechanisms of Converting Dairy Waste into Value-added Products by Microbes’.
Thermochemical processes involve using heat to decompose dairy waste into smaller molecular products through a series of physicochemical reactions. It has been pivotal in transforming dairy waste into valuable products. The processes produce energy outcomes such as biochar, bio-oil, syngas, and combustible gases. Thermochemical processes are classified into combustion, incineration, gasification, pyrolysis, torrefaction, HTC, and HTL (Carlin et al., 2007; Ramola et al., 2020). Hydrothermal methods are suitable for biomass with a high moisture content, while other thermal processes are better for biomass with less moisture content. Factors such as temperature, heating rate, dwell time, pressure, characteristics of biomass, and reactor design influence the sub-processes and chemical reactions that dairy waste undergoes. A detailed description of the HTC, HTL, pyrolysis, and gasification can be found in the review article by Perera et al. (2021).
The HTC method uses water to thermally treat dairy waste at subcritical temperatures (100°C–374°C) and autogenous pressures (2–70 bar; Gómez et al., 2020; Khalaf et al., 2023). This process transforms waste with a high amount of moisture into hydrochar (a solid product enhanced with carbon), a non-condensable gas primarily composed of COx, and a liquid product (process water) containing organic compounds such as carboxylic acid, aldehydes, alkenes, and aromatics (Gao et al., 2018; Pecchi et al., 2020; Silva and Hiibel, 2023). During the HTC process, biomass decomposition happens in four phases through a complicated chemical pathway: hydrolysis, intermediate compound degradation, aromatic production, as well as polymerization (Gómez et al., 2020). The occurrence of other reactions, such as demethylation, Fischer-Tropsch reactions, and transformation, can result in the synthesis of several intermediate products and a complex chemical network (Lentz et al., 2019). In all these processes, the subcritical state of water plays a pivotal role in aiding chemical reactions such as dehydration, decarboxylation, and bond cleavage through hydrolysis (Lachos-Perez et al., 2022). According to Pecchi et al. (2020), the HTC process is mostly exothermic and experiences an enthalpy change of about 1 MJ for every kilogram of dry biomass input. Fig. 2 describes the HTC process.
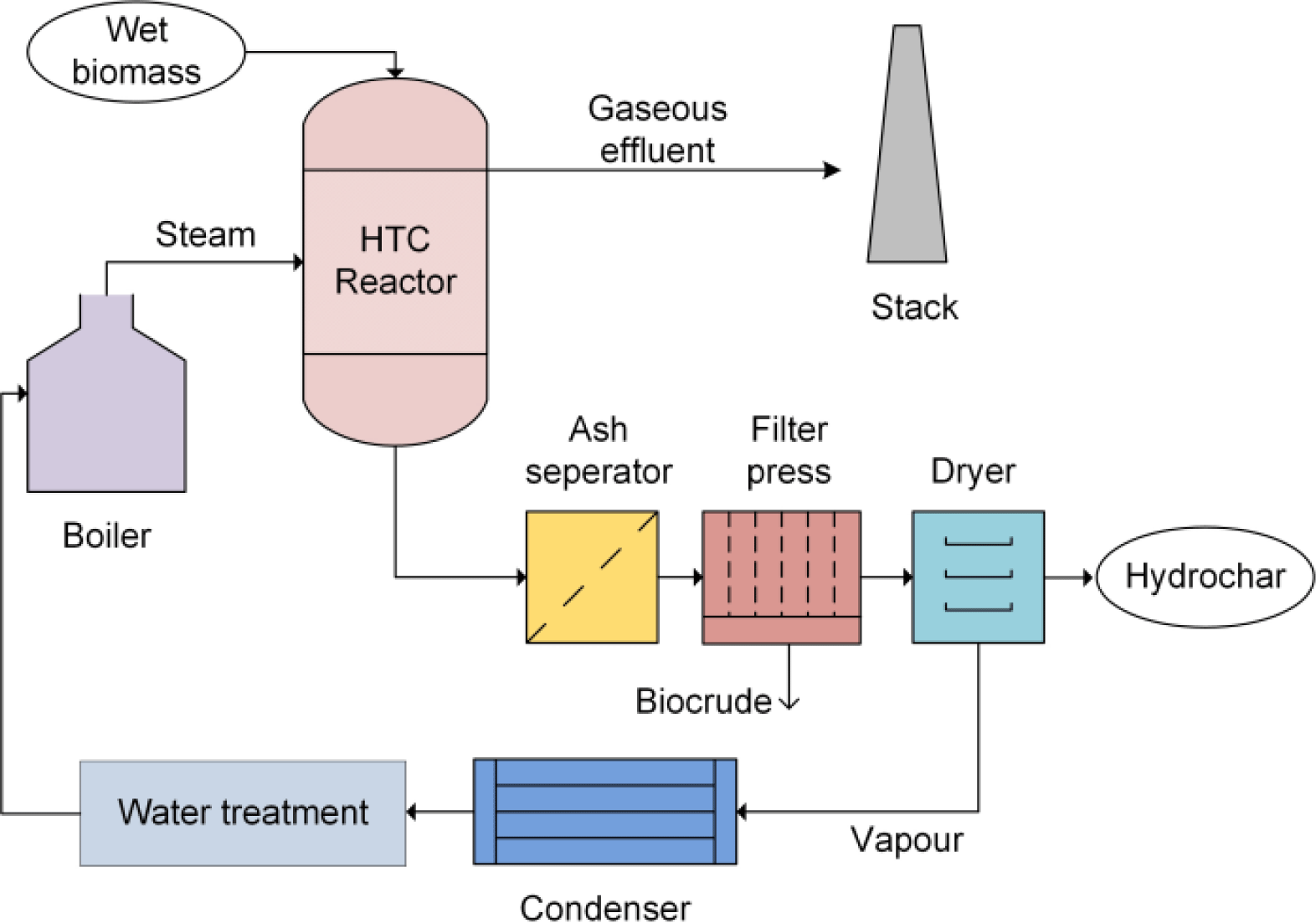
HTC is deemed economical and environmentally friendly since it uses water as the solvent although whey has been suggested as a better substitute (Belete et al., 2021). More so, the properties of water are enhanced at subcritical conditions (100°C–374°C; pressure sufficiently high to maintain the liquid state) making it also act as a catalyst (Alhnidi et al., 2020). In terms of fuel qualities, hydrochar produced via HTC is comparable to bituminous or lignite coal due to its hydrophobicity, better self-binding abilities, low degradability, high carbon content, high homogeneity, and high calorific value (Atallah et al., 2020; Ferrentino et al., 2023). Belete et al. (2021) reported that hydrochars produced via HTC were suitable as energy products as their energy properties were similar to lignite (manure+water) or sub-bituminous coal (manure+whey). However, HTC products’ application depends on the nutrient content and concentration of the nutrients (Alhnidi et al., 2020; Numviyimana et al., 2022). For example, if a hydrochar is intended for energy application, the nitrogen content must be removed to avoid NOx emission during combustion. In contrast, nitrogen removal will unarguably affect hydrochar’s agricultural applications. Also, the liquid product can be used as a fertilizer and a nitrogen or phosphorus precursor for synthesis (Belete et al., 2021). However, when intended for such applications, it must be enriched with the respective element. Therefore, the knowledge of the mechanism of nutrient incorporation and distribution in HTC products is critical for the conversion of dairy wastes using HTC for sustainable applications. Several studies have been devoted to this understanding and various techniques have been advocated. Numviyimana et al. (2022) reported that extraction and struvite precipitation can be used to separate Fe and P into two products with agricultural and chemical usefulness. In their studies, 86.7% of P and 86.6% of Fe were recovered from HTC process water. Belete et al. (2021) studied the HTC of raw and anaerobically digested (AD) manure with water or whey intending to recover energy and nutrients. A net energy gain of 7.4–8.3 MJ/kg dry feedstock and 4.4–5.1 MJ/kg was obtained for HTC of manure with whey and water, respectively while 4.4–5.3 MJ/kg (whey) and 2.3–2.9 MJ/kg (water) was obtained for the combined AD-HTC process. The authors observed that using whey as a liquid for HTC increased the aqueous-phase N-P-K dosages to 3,200, 410, and 7,900 mg/L, respectively indicating suitability as a liquid fertilizer.
Parameters such as temperature, pH, residence time, dairy waste type, waste-to-solvent ratio, and catalyst affect the HTC process. Temperature, pH, and residence time have been found to have the most effect on the distribution of nutrients between hydrochars and the liquid phase. They influence the yield and nutrient distribution. For hydrochar yield, there exists an inverse relationship between temperature and hydrochar yield (Khalaf et al., 2023; Sun et al., 2010) due to high hydrolysis and bond breakages at elevated temperatures. Khalaf et al. (2023) reported a decrease in hydrochar yield from 47.75% to 38.18% and 66.27% to 45.53% at pH of 8.32 and 2.25, respectively upon an increase in temperature from 180°C to 220°C. Similarly, Sun et al. (2010) observed that the yield of hydrochar declined significantly with a rise in temperature from 200oC to 250°C. Generally, the acidic pH is found to favour hydrochar yield. Khalaf et al. (2023) reported that at a temperature range of 180°C–220°C, an increase in the initial acidity pH to 2.25 increased hydrochar yield. A similar observation is reported by Ghanim et al. (2017) and Wang et al. (2017). However, Atallah et al. (2020) in their work on the batch HTC of dissolved air flotation dairy sludge reported that the highest hydrochar and hydrochar energy yields were observed at 250°C, a residence time of 4 h, and a water content of 96 wt.%.
Regarding nutrient distribution, Song et al. (2020) examined the influence of reaction temperature, residence time, and pH on the total concentration and speciation of N and P in pig manure during HTC. It was found that the nitrate content declined at alkaline pH and the acid-extractable fractions in hydrochars decreased significantly with a rise in reaction temperature. Perera et al. (2021) held that, although temperature rise could enhance conversion effectiveness, higher temperatures decrease hydrochar yield but increase the gas yield. The authors advocated moderate temperatures (≤280°C) with increased residence time. According to Ekpo et al. (2016), pH and temperature influence P extraction while N extraction is only affected by temperature. The most favourable conditions for P extraction are acid pH and temperature of about 200°C. The studies of Ekpo et al. (2016) corroborated that of Xiong et al. (2019). Xiong et al. (2019) found that while the content of P in hydrochars increased by 11.3%–33.6% as the reaction temperature increased from 220°C to 280°C relative to raw swine manure, only about 26.9%–39.8% of N was retained in hydrochars after HTC, with the rest entering into the process water. Alhnidi et al. (2020) investigated the fate of carbon and nutrient elements, N, P, and K during the HTC of silage and cattle manure at 180°C, 220°C, 240°C, and 260°C for 3 h reaction time. The results indicated that the potential for nutrient recovery depends mainly on the composition of the feedstock and the pretreatment before HTC.
As advocated by Perera et al. (2021), a balance between the temperature of HTC and the residence time is important for process optimization. The combined influence of these two parameters is usually examined using the severity factor [SF, Eq. (1); Altiparmaki et al., 2022; Lachos-Perez et al., 2022] introduced by Overend and Chornet (1987). Altiparmaki et al. (2022) investigated based on SF, the effect of temperature and residence time on COD, pH, and volatile solids content (phosphates and nitrates concentrations) of DWW. The authors found a synergistic effect between higher residence time and temperature of 180°C–200°C in conformity with the report of Perera et al. (2021) and others.
Where t is the reaction time (min), T is the hydrothermal treatment temperature (°C), and Tref is the reference temperature set by Overend and Chornet (1987) as 100°C.
HTC and HTL are very similar processes as the two technologies utilize mostly water as the solvent, are suitable for biomass with high water content, and are on the composition of the raw material. However, HTC utilizes subcritical water (100°C–374°C; 2–70 bar) with the main mechanism involving hydrolysis, dehydration, decarboxylation, condensation, and polymerization (Lachos-Perez et al., 2022). HTL, on the other hand, uses supercritical water (280°C–370°C; 221 bar; Lachos-Perez et al., 2021; Lachos-Perez et al., 2022). Under supercritical conditions, water self-ionizes to H+ and OH– ions, and its physicochemical properties drastically change (Lachos-Perez et al., 2021; Lachos-Perez et al., 2022). Supercritical water exhibits higher catalytic properties than subcritical water, hence biomass decomposition, solubilization, and fractionation (Lachos-Perez et al., 2022) are highly favoured in HTL. HTL converts high moisture-content dairy waste to products such as bio-oil, char, gases, and water-soluble substances. This technology is still in the developmental stage and is not yet commercially available. However, HTL has the potential to be energy self-sufficient by recycling a portion of the produced bio-oil or char to power the HTL reactor. Additionally, HTL is effective in eliminating pathogens, making it useful for sludge treatment. Nonetheless, at very low solid concentrations, the bio-oil yield from HTL may also be low, and its viscosity may be high. Theegala and Midgett (2012) used a bench-scale HTL system to explore bio-oil production from dairy manure and waste treatment potential. Carbon dioxide (CO2) and Na2CO3 were used as the process gas catalysts, respectively. The process at 350°C process temperature, 1 g of catalyst, and an average higher heating value of 32.16 (±0.23) MJ/kg, produced 4.8 g of acetone soluble oil fractions. The authors observed about 75% of COD reduction in the dischargeable slurry. These results point to the huge potential of HTL as an alternative technology for dairy waste treatment with the potential to generate value-added bio-oils. Chen et al. (2018) investigated the possibility of enhancing dairy manure with various chemicals (NH3·H2O, H3PO4, and glycerol) through HTL for sustainable farming operations. The authors reported that the non-polar compounds (toluene, xylene, and other benzene-containing) increased when NH3·H2O was used. Similarly, the use of H3PO4 was found to enhance the amounts of acids, pyridine, 3-methyl-pyridine, 2,6-dimethyl-pyrazine, 2-cyclopenten-1-ones, and phenols. The authors observed that the biochars possessed high surface area/pore volume and relatively higher N, P, C, and other minerals.
Similar to HTC, the operating temperature, the operating temperature, reaction time, pH, and catalyst affect the quantity and distribution of nutrients in HTL products (Posmanik et al., 2018; Sudibyo and Tester, 2023). Results in the literature indicate that the mass of bio-oil during HTL of dairy wastes increases with an increase in temperature up to 350°C and decreases afterward. Theegala and Midgett (2012) reported an increase of 3.2–3.8 g in the mass of acetone-soluble oil fractions between 250°C and 350°C. Xu and Lad (2008) documented an increment in the mass bio-oil up to 300°C and a decrease when the operating temperature was further increased to 380°C . Similarly, Qu et al. (2003) observed an increase in heavy oil yield between 280°C and 320°C and a decrease at temperature rise to 360°C. Sudibyo and Tester (2023) reported the optimal conditions of pH 4.5, an operating temperature of 354°C, and a reaction time of 21 min. The increase-decrease behaviour is associated with two competing reactions in liquefaction, namely hydrolysis (solubilization and decomposition) and repolymerization (Déniel et al., 2016; Di Domenico Ziero et al., 2020). The solubilization and decomposition occur at temperatures of 200°C–300°C (Di Domenico Ziero et al., 2020; Teri et al., 2014) while the repolymerization reaction takes place at a temperature of °C300°C with the highly reactive organic molecules recombining to form organic compounds such as ketones, aldehydes, amines, hydrocarbons, etc present in the aqueous phase of the bio-oil (Lachos-Perez et al., 2022; Teri et al., 2014). It is important to note that in HTL, the pressure must be maintained within 10 to 25 MPa to avoid water entering the liquid state and preventing the phase change (Lachos-Perez et al., 2022).
Pyrolysis is a thermal process that decomposes biomass in an oxygen-depleted environment at temperatures between 300°C–600°C. It produces pyrolysis vapour, which can be condensed into tar and producer gas, solid char (biochar), and tar (bio-oil). Generally, three types of pyrolysis are known: slow, fast, and ultra-fast (Perera et al., 2021). A detailed description of the operation and kinetic modelling of these three types can be found in Perera et al. (2021). The fast pyrolysis is the most popular and preferred because it produces a high bio-oil yield. It requires a dry feedstock in fine particle form (usually <3 mm) and utilizes a high heating rate and consequently a better operational cost. However, the bio-oil is unstable and requires hydrotreatment and upgrading to be useful (Zhang et al., 2021a). The slow pyrolysis, because of the slower heating rate and longer residence time produces inferior bio-oil in terms of quality and yield relative to that of fast pyrolysis. For example, the bio-oil yield from the Auger continuous reactor slow pyrolysis is 48%–62% while the circulating bed continuous fast pyrolysis could produce bio-oil with a yield of 54%–71% (Perera et al., 2021). The steps involved in pyrolysis include drying, devolatilization, extensive devolatilization, and secondary devolatilization. It is believed that, in decentralized locations, pyrolysis is the most suitable treatment technology for dairy processing sludge since it can be used on a relatively small scale (Maroušek, 2014). Maroušek (2014) claimed that small-scale pyrolysis near feedstock source is an economically viable technology for biochar production from the fermentation residue. However, one of the concerns with thermal conversion technologies including pyrolysis is the discharge of heavy metals and contaminants such as ammonia, hydrochloric, hydrogen cyanide, and hydrogen sulphide gases (Hansson et al., 2004; Johansen et al., 2011). It was reported (Kwapinska et al., 2020; Kwapinska et al., 2021) that 20%–30% of N was left in the biochar after pyrolysis with the greater percentage volatilizing and entering into the gaseous state. Because of such volatilization, post-treatment or flue gas scrubbing is essential.
Kwapinska et al. (2020) demonstrated that pyrolysis can be used in dairy sludge conversion. Generally speaking, the dairy sludge feedstock is problematic for a pyrolysis process because of the high moisture and N contents (Horvat et al., 2019; Kwapinska et al., 2021). According to Kim and Parker (2008), the energy needed for drying is 2–3 times higher than the energy required for low-temperature pyrolysis (300°C–500°C). Nevertheless, pyrolysis is still seen as a better thermochemical treatment technology relative to gasification and incineration (Samolada and Zabaniotou, 2014). Hence, most recent research works are devoted to studying the properties of products derived from the pyrolysis process and how to enrich the products with nutrients to make them suitable for specific applications. It should be mentioned that the application of pyrolysis products just like products of other thermochemical techniques largely depends on the type and composition of nutrients and contaminants present. Kwapinska et al. (2023) examined the attributes of biochars obtained from the slow pyrolysis of dairy processing sludge at 600°C and 700°C and compared them with the European Union Fertilizing Products Regulation (EUFPR). They found that, in terms of hydrogen to organic ratio, chloride, polycyclic aromatic hydrocarbons, and P content, the biochars met the EUFPR. However, of the eight biochars samples analyzed, only two met the N, P, and K requirements for organo-mineral fertilizer. It was found that the biochars complied with the requirements for biochars in the gasification and pyrolysis component category. Horvat et al. (2019) investigated the co-pyrolysis of biological dairy sludge and spruce wood chips at 700°C–770°C and a feedstock feeding rate of 40.9–68.6 kg/h. The aim was to produce pyrolysis gas suitable for an internal combustion engine from a dairy sludge-wood chips blend. The process produced a tar in the range of 7.25–10.98 gtotal tar Nm–3dry raw gas that was rich in N-containing compounds namely, 2-butenenitrile, pyridine, and 1H-pyrrole. Horvat et al. (2019) found that the raw pyrolysis gas contained excessive amounts of 3 and 4+ aromatic ring tars and did not meet the tar limits requirements for internal combustion engines. A thermal tar reformer using air as a reforming agent followed by adsorption was recommended as a tar removal strategy.
The use of gasification for waste conversion, particularly municipal solid waste to energy gas and other valuable products dates back to the 18th century with Berrenrath and Sekundärrohstoff-Verwertungszentrum (SVZ) in Germany being the pioneers (Munawar et al., 2023). The operation gasification temperature can reach 1,000°C and mainly converts waste to syngas (also called synthesis gas or producer gas), a mixture of CO and H2 (Khorasani et al., 2021; Kuo et al., 2014). The overall gasification reaction of a typical biomass (C6H10O5) oxidized with a limited amount of O2 or O2-steam as given by Chang et al. (2023) is summarized in Eqs. (2) and (3). Fig. 3 illustrates the several products that can be derived from the gasification process (Lee et al., 2021).
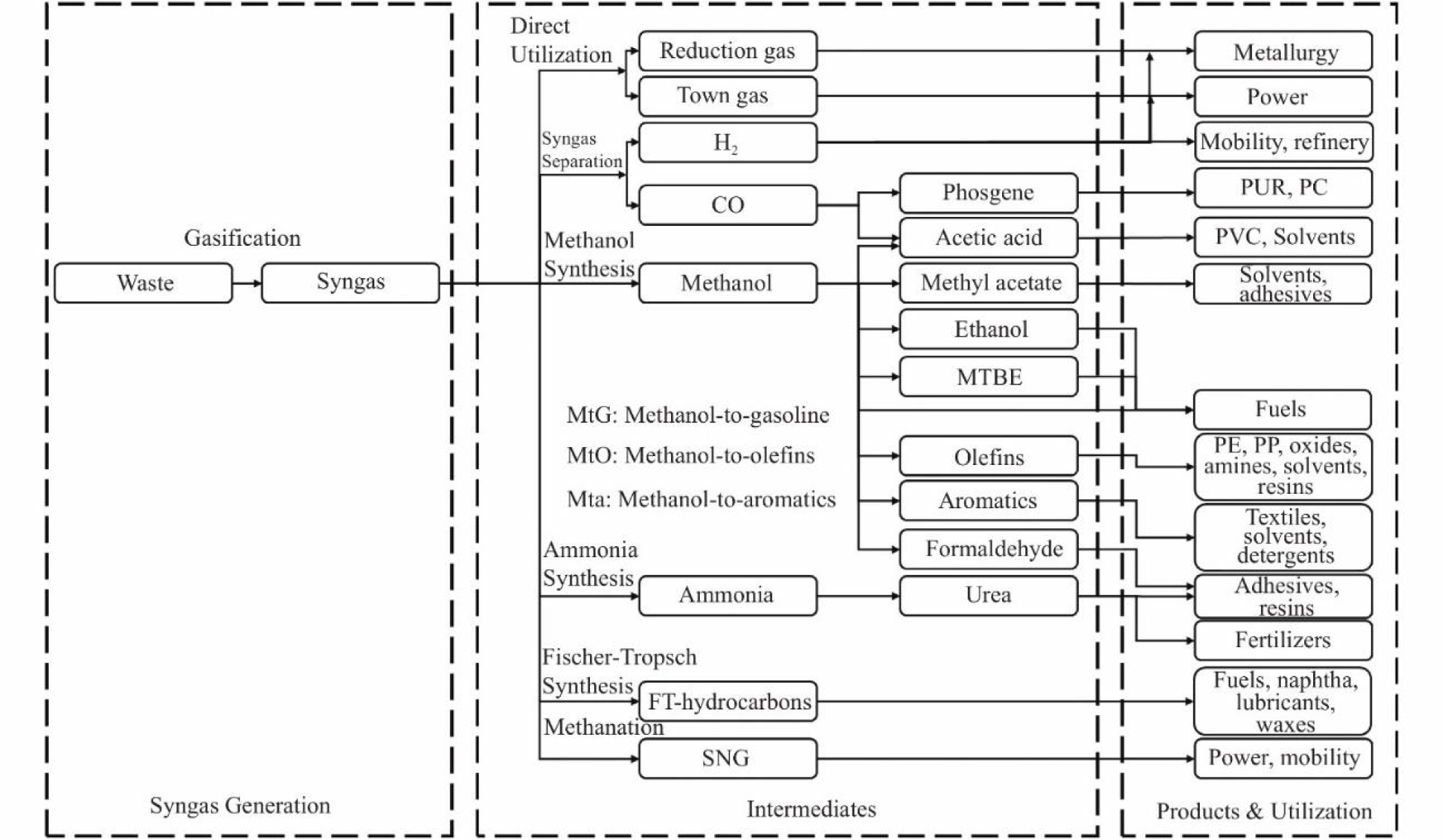
The products may also contain traces of oxygen, sulphur compounds, CO2, CH4, N2, light hydrocarbons, char, ash, and tar. The syngas can be used directly as a fuel or can be converted into energy, liquid fuel, H2, and other compounds with additional value such as methanol, ethanol, methyl tert-butyl ether, and dimethyl ether. In most cases, air, steam, steam-oxygen, air-steam, and oxygen-enriched air are oxidizing agents. The syngas’ quality depends on the type of oxidizing agent used and the conversion efficiency is largely affected by temperature. Young and Pian (2003) in their work on the gasification of dairy biomass wastes into fuel gas in a multistage enthalpy extraction technology gasifier that uses a high-temperature preheated air as the oxidizer found that the gross heating value of the produced gases as well as the gross and net gasification conversion efficiencies increased with a rise in air temperature. Raman et al. (1980) investigated the influence of a reactor operating temperature on the product gas yields and composition of gasified feedlot manure. The gasification was performed in a fluidized bed gasifier where the combustion product of propane and air was the fluidization gas and silica sand served as the bed material. It was found that the gas yields (CO, H2, and CH4) and the heating value improved with a rise in the operating temperature.
In the gasification of dairy waste, a stoichiometric air-to-fuel ratio typically ranges from 6:1 to 6.5:1. However, due to conditions of combustion being below stoichiometry, the real air-to-fuel ratio often lies between 1.5:1 and 1.8:1. Thanapal et al. (2012), in an attempt to improve gas heat values, gasified dairy biomass in a medium with enriched oxygen varying from 24% to 28% oxygen on a volume basis. The influence of the enriched air mixture, equivalence ratio, and steam fuel ratio on the efficiency of a fixed bed gasifier was examined. It was observed that the peak temperature and CO production appreciated with an increase in O2 concentration in the incoming stream. They noted that the gases produced using a mixture of CO and O2 exhibited a higher heating value than that of air and enriched air gasification.
When the oxidizing agent is supercritical water, the technique is often called supercritical water gasification or hydrothermal gasification (Nanda et al., 2015; Yildirir and Ballice, 2019). Hydrothermal gasification has been widely used to gasify dairy wastes. This technique, an alternative to anaerobic digestion, was developed to treat feedstocks with up to 80% water content (Munawar et al., 2023). Because of the unique properties of supercritical water, hydrothermal gasification produces a high yield of syngas with low chars and completes the decomposition of wet feedstocks within a short time (Munawar et al., 2023). Other advantages of supercritical water gasification include minimal corrosion issues due to the dissolution of corrosive ions such as chlorides and low amounts of tars (Munawar et al., 2023). Additionally, the transportation of the syngas is convenient because of the high-pressure nature of supercritical water gasification.
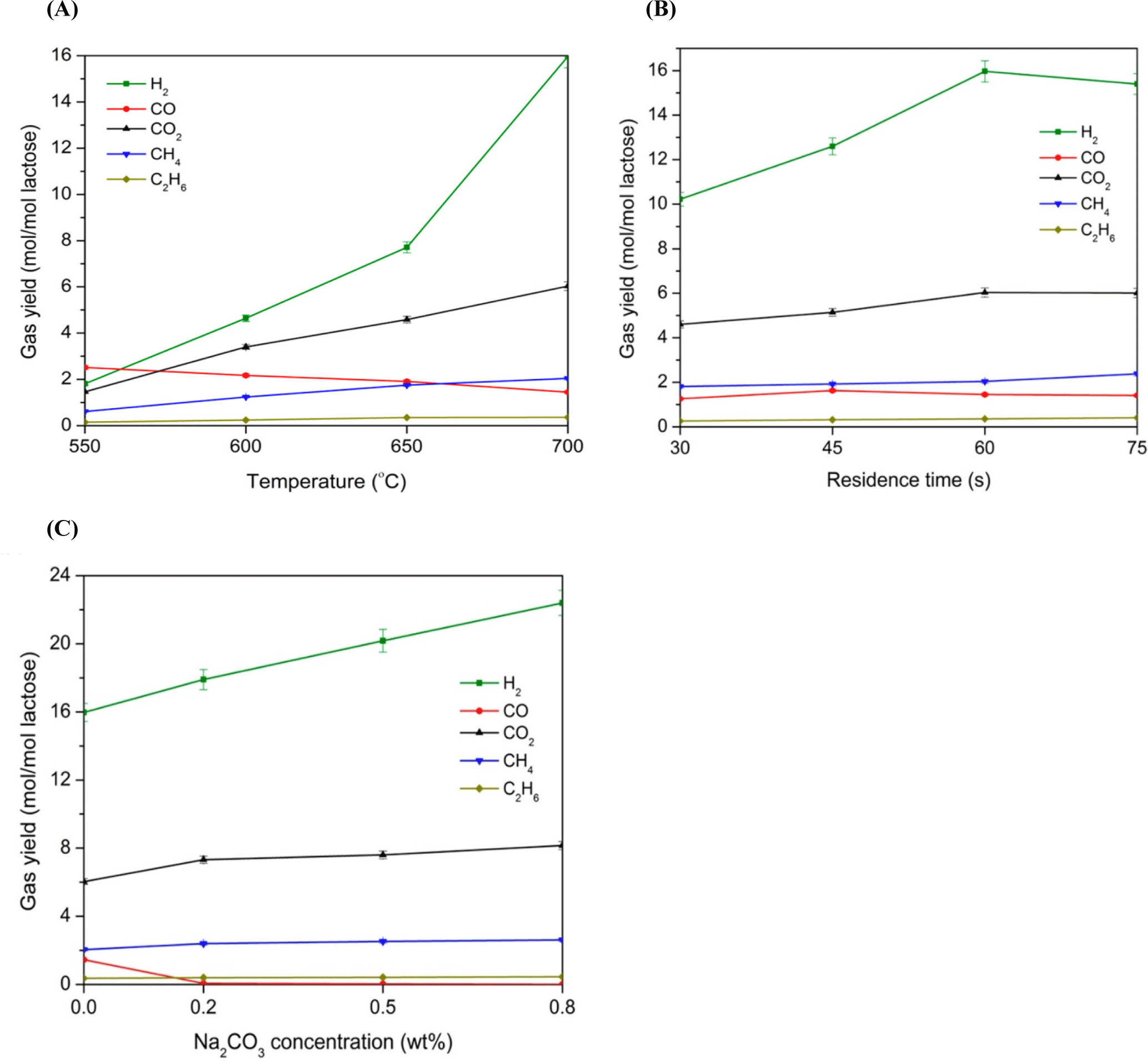
As previously mentioned, the operating temperature significantly affects the product yield of thermochemical processes. For the hydrothermal gasification of dairy wastes, a temperature of 400°C–700°C is found to favour the gasification yield. For example, Nanda et al. (2015) subjected lactose-based modeled DWW to hydrothermal gasification and examined the influence of operating temperature and catalyst (Na2CO3) on the yield of produced gases. The yield of the gases increased when the operating temperature and residence time were raised and 0.8 wt.% of the catalyst was used (Fig. 4). In a similar investigation, wet sludge obtained from the biological treatment of textile and leather wastewater was hydrothermally gasified at the temperature range of 400°C to 550°C and reaction time ranging from 0–60 min (Yildirir and Ballice, 2019). The findings showed that product yield almost doubled when the operating temperature was raised from 400°C to 550°C. Khorasani et al. (2021) studied the hydrothermal gasification of real DWW (cheese-based or whey) in a batch reactor. To examine the influence of operating temperature, time, catalyst, and additives on H2 gas production, the temperature and time were varied between 350°C–400°C and 30–60 min, respectively. MnO2 and MgO were deployed as the catalysts and formic acid as an additive at 1 wt%, 3 wt%, and 5 wt% concentrations. It was observed that increased temperature and elongated residence time enhanced gasification efficacy and H2 production. At optimum conditions of 400°C and 60 min, gas yield of 1.36 mmol/gr dry ash free was achieved. Furthermore, the use of MnO2 at 1 wt.% and formic acid at 5 wt.% were found to be beneficial producing the highest H2 content of 35.4% and gasification efficiency of 32.22%. Nevertheless, as in the treatment of wet feedstock using other thermochemical techniques, the large amount of energy needed to dry the damp feedstock remains an issue in the hydrothermal gasification process.
Several factors namely temperature, heating rate, dwell time, pressure, characteristics of biomass, and reactor design influence thermochemical processes (Atallah et al., 2020; Carlin et al., 2007). The influence of some of the parameters has been highlighted in the preceding sections. This section gives a snapshot of the optimal conditions. Optimizing the thermochemical processes of dairy waste valorization requires careful consideration of these factors to maximize product yields, energy efficiency, and economic viability (Atallah et al., 2020; Carlin et al., 2007). Experimental studies and computational modeling can be employed to assess the effects of these factors and optimize process conditions for specific feedstocks and desired products.
Temperature: Temperature plays a crucial role in thermochemical processes as it determines the extent of decomposition and the types of products formed. In pyrolysis, low temperatures (300°C–400°C) favour the production of biochar, while higher temperatures (400°C–800°C) promote the formation of gases such as syngas (CO+H2) and bio-oil (Bhaskar et al., 2011; Carlin et al., 2007; Zhang and Zhang, 2019). Gasification typically requires higher temperatures (800°C–1,000°C) to convert biomass into synthesis gas (syngas) composed of CO, H2, CO2, and CH4 (Bhaskar et al., 2011; Carlin et al., 2007; Zhang and Zhang, 2019). Liquefaction temperatures vary depending on the specific process but typically range from 250°C to 500°C, with higher temperatures favouring liquid fuel production (Lachos-Perez et al., 2022; Posmanik et al., 2018).
Heating rate: Heating rate influences the kinetics of thermochemical reactions. Higher heating rates generally result in faster reactions and shorter residence times (Atallah et al., 2020; Bhaskar et al., 2011). Rapid heating rates can enhance gas yields and increase the efficiency of the process. However, excessively high heating rates may lead to incomplete conversion or undesired side reactions.
Dwell time: Dwell time refers to the duration that biomass spends at reaction temperatures within the reactor. Longer dwell times often result in the complete conversion of biomass and higher yields of desired products. Atallah et al. (2020) noted that the optimal conditions for hydrochar production in batch HTC of dissolved air flotation dairy sludge is a temperature of 250°C, a residence time of 4 h, and a water content of 96 wt.%. However, excessively long dwell times can increase energy consumption and operational costs (Atallah et al., 2020; Jha et al., 2022).
Pressure: Pressure affects the thermodynamics and kinetics of thermochemical reactions. Higher pressures can increase the yield of certain products, such as liquid fuels, by suppressing vaporization and promoting secondary reactions (Bhaskar et al., 2011; Jha et al., 2022). Gasification processes typically operate at elevated pressures to improve gas yields and enhance process efficiency.
Characteristics of biomass: The composition and properties of dairy waste biomass, including moisture content, ash content, and organic composition, significantly influence thermochemical conversion processes (Bhaskar et al., 2011; Jha et al., 2022). High moisture content can decrease the heating value and efficiency of the process (Atallah et al., 2020), while high ash content can lead to reactor fouling and corrosion. The presence of contaminants such as salts and heavy metals may also impact process performance and product quality (Bhaskar et al., 2011; Jha et al., 2022).
Reactor design: Reactor design plays a critical role in determining heat and mass transfer rates, residence times, and the distribution of products. Various reactor configurations, such as fixed-bed, fluidized bed, and entrained flow reactors, have different advantages and limitations (Bhaskar et al., 2011; Jha et al., 2022). Reactor design should be optimized to ensure efficient heat transfer, adequate mixing, and minimal tar formation or reactor fouling (Azoumah et al., 2007). More information on the impact of these factors on the thermochemical processes can be found in Refs (Hawwash et al., 2020; Rahimi et al., 2022).
The biological processes involve converting dairy industry waste into valuable resources using living organisms, primarily microorganisms such as bacteria, fungi, and algae (Kalia et al., 2000). This process mitigates environmental pollution and produces valuable products such as biogas, compost, enzymes, single-cell protein (SCP), lactic acid, and bioplastics. The primary biological processes are anaerobic and aerobic (Goli et al., 2019). Other sub-biological processes include lactic acid fermentation, SCP production, enzyme production, polyhydroxyalkanoates (PHAs) production, biohydrogen production, and microbial fuel cells (MFCs; Goli et al., 2019; Joshiba et al., 2019). Each process utilizes different microorganisms and metabolic pathways to achieve these conversions, offering diverse applications ranging from renewable energy production to soil improvement and the creation of biodegradable materials (Joshiba et al., 2019). Lactic acid fermentation employs lactic acid bacteria (LAB) to convert lactose in dairy waste into lactic acid. Firstly, the dairy waste is inoculated with specific strains of LAB. Under anaerobic conditions, the bacteria ferment lactose into lactic acid and purified for industrial applications (Goli et al., 2019). SCP production involves cultivating microorganisms on dairy waste to produce biomass rich in protein, which can be used as animal feed. The dairy waste is first treated to optimize nutrient availability. Afterwards, microbial biomass is harvested, dried, and processed into SCP and used as a protein supplement in animal feed (Koukoumaki et al., 2024; Raziq et al., 2020).
PHAs are biodegradable polymers produced by bacterial fermentation of organic substrates, including dairy waste. Here bacteria are grown on dairy waste substrates. The bacteria further synthesize and accumulate PHAs under nutrient-limiting conditions. The PHAs are extracted from bacterial cells and then purified. Biohydrogen production on the other hand, involves the microbial conversion of dairy waste into hydrogen gas, a clean energy source. The dairy waste is pre-treated to enhance its fermentability. Subsequently, hydrogen-producing bacteria ferment the waste anaerobically to produce hydrogen gas which is collected and purified for use (Colombo et al., 2019). Additionally, MFCs use microorganisms to directly convert organic matter in dairy waste into electricity. In the first step, microorganisms oxidize organic matter, releasing electrons. The electrons flow through an external circuit to the cathode, generating electricity. Bio-electrochemical systems (BES) offer an innovative approach to waste treatment while generating valuable products, such as electricity and hydrogen, making them a crucial component of the circular economy (Jung et al., 2020). BES utilize electrochemically active microbes to break down organic matter, resulting in electron transfer from the anode to the cathode. The efficiency of energy output is determined by the number of electrons accumulated and transferred to the anode (Adesra et al., 2021; Do et al., 2020; Sambavi et al., 2020). Microbial Electrosynthesis, enzymatic biofuel cells, microbial desalination cells, microbial reverse-electrodialysis cells, microbial solar cells, microbial electrolysis cells and MFCs, are some examples of BES. MFCs are the most studied and advanced BES (Ivase et al., 2020; Mishra et al., 2020). Of all these processes, the most developed and commonly utilized biological processes are the anaerobic and aerobic processes.
AD is a well-established process that decomposes organic matter in the absence of oxygen to produce biogas and digestate. Biogas mainly consists of methane (CH4) and CO2 while digestate is a nutrient-rich fertilizer (Bella and Rao, 2021; Goli et al., 2019). The biogas so produced can be used as a combustion gas to operate a generator, producing heat and electricity. It can also be used as an alternative to natural gas for cooking gas, as fuel (bio-methane), and for chemical synthesis (Goli et al., 2019). AD utilizes a 4-steps process involving hydrolysis, acidogenesis, acetogenesis, and methanogenesis. During hydrolysis, the complex organic polymers (proteins, fats, carbohydrates) are broken down by hydrolytic bacteria into simpler molecules (amino acids, fatty acids, sugars) through the secretion of some extracellular enzymes (Patel et al., 2021). The simpler molecules are converted into volatile fatty acids (VFAs), alcohols, hydrogen, and CO2 in a process called acidogenesis. Further conversion of the products into acetic acid, hydrogen, and CO2 is called Acetogenesis while in methanogenesis, methanogenic archaea convert acetic acid and hydrogen into methane and water (Bella and Rao, 2021). The stages of Anaerobic digestion are shown in Fig. 5. Acidogenesis usually occurs at a faster rate than other stages with a regeneration time of less than 36 hours. The effectiveness of these processes also depends on the type of anaerobic digestion process applied.
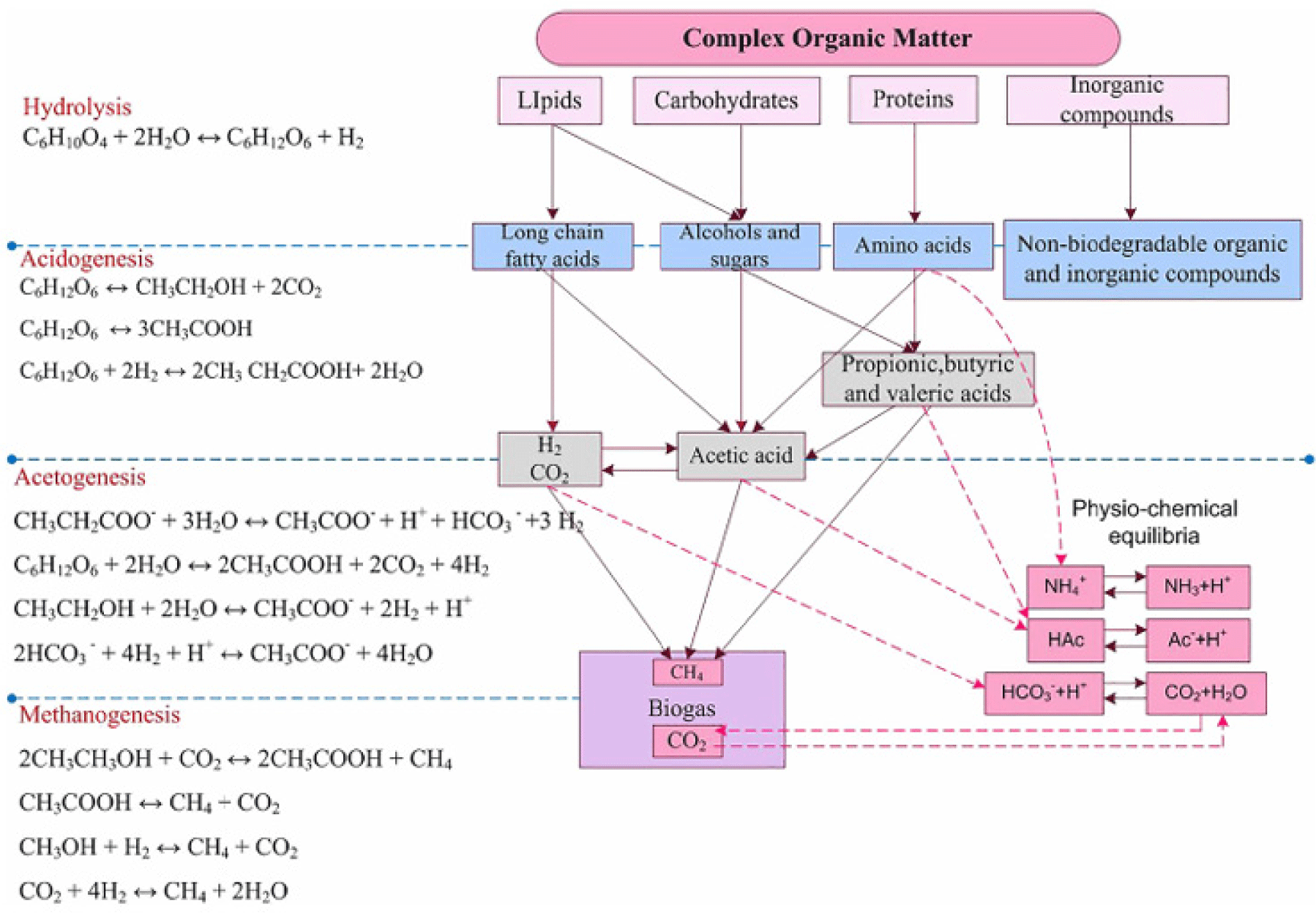
There are various anaerobic biological treatment methods. They include up-flow anaerobic sludge blanket reactors (UASB), up-flow anaerobic filter reactor (UAFR), anaerobic hybrid reactors (AHR), anaerobic sequencing batch reactors (ASBR), completely stirred tank reactor, membrane anaerobic reactor, fixed bed reactor, Anaerobic lagoon/ponds, and Fluidized bed/expanded bed reactor. In dairy waste treatment, UASB, UAFR, AHR, and ASBR are commonly used (Bella and Rao, 2021).
UASB: UASB reactors are useful to treat large volumes of wastewater in a short period. They comprise a single tank setup and are considered beneficial for AD processes due to the formation of anaerobic granules in the reactors. The sludge production is less and can be operated at a high organic loading rate (OLR) and low temperatures (Han et al., 2018). In a study by Kavitha et al. (2013), 77% COD and 87% BOD removal efficiency were reported when DWW was treated in a UASB reactor. The inlet COD concentration ranged from 1,090 to 1,415 mg/L and BOD values between 1,015 and 1,370 mg/L (Kavitha et al., 2013). Vidal et al. (2019), applied a UASD reactor at two different OLRs (3.94 and 8.15 g COD/L/d) for the treatment of slaughterhouse wastewater and achieved 70% COD removal efficiency for the highest OLR, and low efficiency of suspended solid removal. However, treatment of the anaerobic effluents by solar photoelectron-Fenton process resulted in 88% and 72% COD removal for initial concentrations of 195±14 mg/L and 867±52 mg/L respectively, indicating the potential for an integration process (Vidal et al., 2019).
The robustness of the UASB technology was also demonstrated by Vassalle et al. (2020), by co-treating raw sewage and microalgal biomass from a high-rate algal pond. The results showed an overall removal of 65% COD and 61% N-NH4 with 25% methane yield (Vassalle et al., 2020). Moreover, Mainardis et al. (2020) studied the technological advances in UASB and concluded that an increased full-scale application of UASB technology is desirable to achieve a circular economy and sustainability scopes, with efficient biogas exploitation, fulfilling renewable energy targets, and GHG emission reduction.
Due to the major downside related to the traditional UASB reactor, which is that of the long period for start-up amount, a modified UASB called up-flow anaerobic sludge-fixed film (UASFF) reactor was developed and applied as a granular sludge bioreactor for the fast-biological conversion of organic concern biogas with the aid of aggregative microbic association. This shortens the start-up amount at a low Hydraulic Retention Time (Sivaprakasam and Balaji, 2021). In the study by Sivaprakasam and Balaji (2021), the influence of OLR in the biodegradation performance of a lab-scale hybrid up-flow anaerobic sludge fixed film (UASFF) reactor for treating synthetic dairy industry wastewater was evaluated. The lab-scale UASFF reactor was designed and fabricated for an effective volume of 13 L with Fujino spiral as packing media at the top of the reactor and operated in continuous mode. The reactor was operated for different OLRs ranging from 0.28 kg COD/m3. d to 2.33 kg COD/m3. d by varying the influent COD concentrations of 8,500 mg/L, 10,000 mg/L, 11,500 mg/L, 13,000 mg/L and 14,500 mg/L under steady state conditions. The results varied between 70.60% and 93.60% COD removal efficiency. The authors found that an increase in the inflowing concentration reduces the COD removal potency.
UAFR: For the treatment of DWW having a low concentration of suspended solids, studies have shown that the start-up performance with UAFR is less affected by temperature variations (between 20°C and 30°C) and hydraulic retention time (HRT). Consequently, Ince (1998), applied the UAFR for 3 months at an HRT of 20 h and achieved 85% and 90% removal efficiency of COD and BOD respectively. Also, a pilot-scale study conducted by Grobicki and Stuckey (1991) using synthetic DWW in a single and double-stage UAFR, recorded almost the same trend of results for both reactors having been operated at an HRT of 2 days and substrate concentration ranging from 3 to 12 g COD/L (Grobicki and Stuckey, 1991). An industrial-scale study that treated wastewater discharged from raw milk quality control labs with UAFR operated for 2 years, recorded more than 90% COD removal with full-fat degradation (Omil et al., 2003). These studies opined that the UAFR method for AD processes is less affected by the hydraulic or organic load, long-time retention of biomass, and has a high methane yield in a small reactor volume (Grobicki and Stuckey, 1991; Ince, 1998; Omil et al., 2003).
Aerobic treatment processes are biological processes that utilize oxygen-requiring microorganisms to decompose organic matter into valuable products. It involves microbial waste oxidation and breakdown in the presence of oxygen. The aerobic treatment of dairy waste involves various methods that use oxygen-requiring microorganisms to decompose organic matter. These methods effectively reduce the organic load, control odors, and convert waste into valuable products such as compost, biofertilizers, and treated effluent suitable for discharge or reuse (Joshiba et al., 2019; Kalia et al., 2000). The primary aerobic treatment methods employed in the dairy industry include activated sludge process (ASP), rotating biological contactors (RBCs), sequencing batch reactor (SBR), membrane bioreactor (MBR), conventional or percolating/ trickling filters, aerobic digestion, and aerobic composting. Three principal steps are involved in all cases: oxidation, degradation, and stabilization. At the oxidation step, the microorganisms use oxygen to oxidize organic matter, producing CO2, water, and biomass. The complex organic compounds are then broken down into simpler molecules through enzymatic actions at the degradation stage, which are subsequently converted into stable end products, reducing their potential to cause environmental harm (Ashekuzzaman et al., 2019; Khalaf et al., 2021; Rahul Krishna et al., 2022).
ASP: Activated sludge systems treat DWW through a combination of aeration and microbial action. In the process, DWW is first aerated to maintain dissolved oxygen levels, promoting microbial growth and organic matter degradation (Ashekuzzaman et al., 2019; Rahul Krishna et al., 2022). Afterward, biomass (activated sludge) is separated from treated effluent and part of the sludge is recycled back into the aeration tank. The excess sludge is then treated and disposed of or used for land application. The treated effluent can be discharged into water bodies or reused for agricultural or industrial purposes (Joshiba et al., 2019).
The ASP is one of the most used processes in organic matter removal due to its higher shock resistance, lower toxicity, improved sludge settleability, and high efficiency in biomass recovery (Rahul Krishna et al., 2022). As previously mentioned, DWW contains organic matter such as lactose, proteins, fats, and so on, hence can be biodegraded by microorganisms present in activated sludge. Various studies have shown the successful application of ASP for DWW treatment. Donkin and Russell (1997) reported successful removal of 90% COD with milk powder and butter wastewater. However, removing phosphorus compounds from the wastewater was less reliable and appeared to be sensitive to environmental variations (Donkin and Russell, 1997). Also, the effect of varying retention time in the activated sludge system was investigated by Lateef et al. (2013), and the removal efficiencies of COD and BOD were 96% within five days. Increased retention time did not notably affect the BOD and COD removal rather it enhanced uniformity in the ponds, in which the action keeps the immune system from organic shocks. It also lowered sludge production due to the digested part of microorganisms in this section. However, the bulking phenomenon due to the lack of sedimentation created excessive foam and toxic materials (Lateef et al., 2013). On the whole, ASP offers the advantages such as ease of installation and operation, free from odor and light footprint. On the other hand, it has limitations such as low effluent quality, higher sludge production, high energy consumption, bulkiness, foam production, precipitation of iron and carbonates, and a decrease in efficiency during winter (Goli et al., 2019).
RBCs: RBC is made up of several discs that are fastened to a shaft. A multi-unit system with three to six sets of RBCs can be used to accomplish adequate reduction. Three-stage RBCs are efficient and effective. However, owing to its high fat, oil, and grease concentration, aerobic filters may have trouble processing high-strength wastewater, such as dairy effluent. As a result, there may be significant biofilm formation, biomass sloughing, and eventual decrease in productivity (Slavov, 2017). RBC utilizes rotating discs or media to support the growth of biofilms consisting of microbial communities capable of degrading organic matter (Rahul Krishna et al., 2022). As DWW flows over the rotating discs, organic compounds are biodegraded by microorganisms within the biofilm. The rotation of the discs enhances oxygen transfer and mixing, promoting aerobic microbial metabolism and organic matter removal. Treated effluent from RBCs can be further processed for valorization purposes, such as nutrient recovery through struvite precipitation or energy generation through anaerobic digestion (Rahul Krishna et al., 2022). Many reports have shown the effective use of RBC in dairy waste treatment.
In a study by Rusten et al. (1992), the RBC process suggests various superiority over the ASP in dairy effluent treatment. The study presented a COD removal efficiency of 85% with an OLR of 500 g COD/m3 hour when treating dairy effluent. Powar et al. (2023) applied RBC for the treatment of dairy waste effluents and achieved 91.5% BOD removal and 89.3% COD reduction. The treated waste was then applied for irrigation of soy and corn crops and it exhibited an increase in organic matter from 0.262% to 0.395% for corn and 0.416 for soya. The available total nitrogen, phosphorus, and potassium nutrient composition also increased and proved to be more efficient compared to the regular groundwater used to irrigate the crops (Powar et al., 2023). Most reports have shown the advantages of RBC to include low sludge production, high removal efficiency. Low power input, ease of operation, low maintenance, less operator attention, lower operating costs, well-controlled against organic shocks, low space requirement, no risk of channeling, while the disadvantages include odor needs for a permanent skilled technical operator for operation and maintenance purposes, needs for protection against sunlight, wind, and rain (especially against freezing in cold climates), considerable investment, operation, and maintenance costs, non-availability of contact media at the local market, needs for continuous electricity.
SBR: The SBR is a tank-based technology that is used to remove undesirable substances from wastewater. Tanks that serve as batch reactors are filled at various intervals to allow aeration and sedimentation processes to take place simultaneously. Since equalization, aeration, and sedimentation all occur in the same tank, there is no need for a clarifier. It can therefore process waste with a higher solids content and requires less expenditure, making it a cost-effective option. Several operating factors, including hydraulic retention time, mixed liquor-suspended solid, organic loading, dissolved oxygen content, phase duration, temperature, wastewater strength, and pH, affect how effectively SBR treatment works (Birwal et al., 2017). SBRs offer a flexible and efficient way to treat DWW by providing sequential treatment stages within a single reactor. The SBR cycle typically includes filling, aeration, settling, and decanting phases. This creates room for the optimization of treatment conditions. During the aeration phase, aerobic microbial activity is promoted, leading to the biodegradation of organic compounds present in the DWW. The settling phase allows for the separation of treated wastewater from the biomass, which can be recycled back into the reactor to maintain microbial populations and treatment efficiency. Treated effluent from SBR can undergo further processing for valorization purposes, such as nutrient recovery or biogas production (Ashekuzzaman et al., 2019; Khalaf et al., 2021; Rahul Krishna et al., 2022). SRB offers a wide range of advantages such as easy-to-modify cycles, small footprint, wider wastewater strength variations, high removal efficiency, the capability of achieving nitrification, de-nitrification, and phosphorous removal, cost-effective, low flow applications, wide operation flexibility, minimal sludge bulking, minor operation and maintenance issues. On the other hand, it exhibits the following limitations: high energy consumption, difficulty in adjusting cycle times for small communities, and frequent sludge disposal.
Aerobic digestion and composting: Aerobic digestion is used to stabilize solid dairy waste and reduce its volume by decomposing organic matter in the presence of oxygen (Goli et al., 2019). Firstly, the solid dairy waste is continuously aerated to provide oxygen for microbial activity. Microorganisms degrade the organic matter, converting it into CO2, water, and stabilized biomass. The stabilized biomass can be used as a biofertilizer or soil amendment (Goli et al., 2019). A comprehensive comparative review of aerobic and anaerobic dairy waste treatment can be found in Goli et al. (2019). Aerobic composting, on the other hand, is a controlled biological process that converts organic waste into a stable, humus-like product through aerobic decomposition (Zhang et al., 2020; Zhang et al., 2021b). This is achieved by mixing the dairy waste with bulking agents (e.g., straw, wood chips) to enhance aeration and carbon-to-nitrogen ratio (Zhang et al., 2021a). The organic matter is subsequently decomposed by microorganisms, generating heat and reducing pathogen levels. The compost is finally cured to stabilize the organic material. The resulting compost can be used as a soil conditioner and organic fertilizer.
A comprehensive integration of relevant thermochemical methods along with biological dairy waste treatment processes using various biochemical and remediation techniques can provide an innovative approach to effective dairy waste management and economic benefits for the expansive and ever-growing global dairy industry (Adesra et al., 2021; Lin et al., 2021). Carbonaceous waste can be converted into hydrochars and biochars using waste-to-resource conversion processes like HTC and pyrolysis. However, neither approach singlehandedly offers a comprehensive solution to dairy waste management (Lin et al., 2021). HTC is the finest practice for treating wet biomasses with municipal solid waste and cow dung since it includes heating a solid in subcritical water under autogenous pressure. The hydrochars produced, however, have limited surface areas, which makes them inappropriate for soil additions and other methods of solid carbon valorization. Drying wet feedstocks requires a lot of energy, hence pyrolysis, which includes heating at high temperatures in an inert atmosphere, is better suited for dry wastes like agricultural residues. Nonetheless, because of the low nutritional concentration in most pyrolyzed biomasses, fertilizers must be employed in addition to bio-chars when utilized as soil additions. Both HTC and pyrolysis generate liquid biofuels, as well as a gas stream containing light volatiles and syngas components such as CH4, C2H6, CO, CH4, and H2 (Cantrell et al., 2007; Lucian et al., 2018). Therefore, integrated processes that combine HTC and pyrolysis are advantageous for the valorization of both wet and dry dairy wastes, resulting in the production of nutrient-rich soil amendments and liquid biofuels (Cantrell et al., 2007; Zornoza et al., 2016).
Furthermore, biological processes such as aerobic and anaerobic treatments generate sludge, which typically contains substantial organic matter and requires further processing before disposal (Adesra et al., 2021). After feeding sludge into a digester to continue disintegration and stabilization, the dewatered sludge can be utilized as compost, disposed of in a landfill, or spread on land.
AHR can offer the benefits of both attached and suspended growth systems. For example, a typical type of AHR called Bio-nest offers benefits such as good sludge mixing, longer sludge retention time, and less wash-out of sludge. This AHR setup is made by combining properties of both the UASB reactor and UAFR (Gonzalez-Tineo et al., 2020). Rajesh Banu et al. (2008) treated DWW by using anaerobic and solar photocatalytic oxidation methods. The integration of anaerobic and solar photocatalytic treatment resulted in 95% removal of COD from the DWW. The anaerobic treatment was carried out in a laboratory-scale hybrid UASB with a working volume of 5.9 L. It was operated at an OLR varying from 8 to 20 kg COD/m3 day for 110 days. The maximum loading rate of the anaerobic reactor was found to be 19.2 kg COD/m3 day and the corresponding COD removal at this OLR was 84%. Kongsil et al. (2010) applied an anaerobic Bio-nest and an aerobic entrapped mixed microbial cell integrated configuration for valourization of DWW at 25°C–30°C recorded a total COD removal efficiency of 85%–95% at an OLR of 0.5–0.9 g/L/day with an average CH4 content of 68%. The medium-strength wastewater was found to be better treated than high-strength wastewater. Integrated aerobic-anaerobic reactors, coupling a UASB section and a packed bed reactor, were investigated for swine wastewater treatment, with a progressive OLR increase, allowing nitrogen removal in the final aerobic phase (Gonzalez-Tineo et al., 2020).
Processes and Mechanisms of Converting Dairy Waste into Value-added Products by Microbes
Microbes are crucial in converting dairy waste into value-added products through various biochemical processes. Different groups of microbes contribute to waste valorization through mechanisms such as fermentation, anaerobic digestion, and biosynthesis (Awasthi et al., 2022; Liguori et al., 2013). The microbial consortia in dairy waste have diverse metabolic capabilities that can be harnessed to convert waste into value-added products as illustrated in Fig. 6. By understanding and optimizing microbial processes, such as fermentation, anaerobic digestion, and biosynthesis, it is possible to enhance the efficiency and sustainability of dairy waste valorization (Awasthi et al., 2022; Liguori et al., 2013). A summary of various microbes involved in dairy waste conversion is given below.
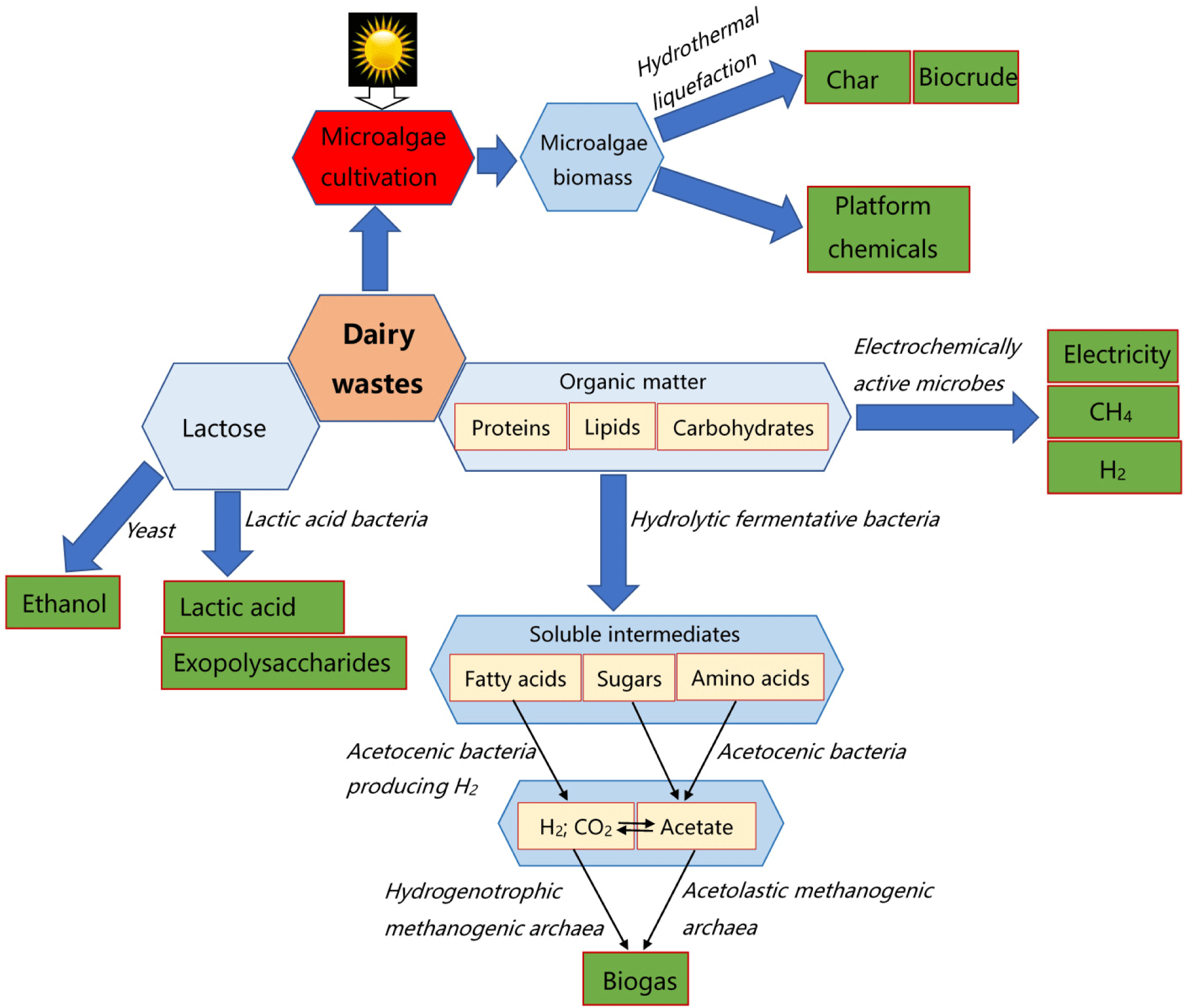
LAB: LABs are commonly found in dairy waste and are known for their ability to ferment lactose, a predominant sugar in dairy waste, into lactic acid (Abedi and Hashemi, 2020; Ghosh and Ghosh, 2013). Lactose fermentation by LAB not only helps in reducing the pH of the waste, inhibiting the growth of spoilage organisms, but also produces lactic acid, which has applications in the food, pharmaceutical, and cosmetic industries (Abedi and Hashemi, 2020; Ghosh and Ghosh, 2013). Certain LAB strains can also produce exopolysaccharides during fermentation, which have functional properties and can be used as thickening agents or stabilizers in various products (Abedi and Hashemi, 2020; Ghosh and Ghosh, 2013).
Methanogenic archaea: Methanogenic archaea are responsible for anaerobic digestion, a process where organic matter in dairy waste is broken down into biogas (mainly methane and CO2) in the absence of oxygen. In anaerobic digestion, complex organic compounds such as proteins, lipids, and carbohydrates in dairy waste are hydrolyzed by hydrolytic bacteria into simpler compounds like fatty acids, amino acids, and sugars. Methanogenic archaea then metabolize these intermediate products to produce methane and CO2, which can be captured and utilized as a renewable energy source (Awasthi et al., 2022; Liguori et al., 2013).
Acetogenic bacteria: Acetogenic bacteria are involved in the production of acetate from various organic substrates, including VFAs generated during anaerobic digestion. These bacteria play a crucial role in the syntrophic oxidation of VFAs, where they convert VFAs such as acetic acid into acetate, hydrogen, and CO2. Acetate produced by acetogenic bacteria can serve as a precursor for the biosynthesis of value-added chemicals such as ethanol, butanol, and other organic acids (Awasthi et al., 2022; Liguori et al., 2013).
Yeasts and fungi: Yeasts and fungi can ferment sugars and other carbohydrates present in dairy waste into ethanol, organic acids, and other metabolites. Certain yeast species, such as Saccharomyces cerevisiae, are widely used in ethanol fermentation, converting sugars into ethanol and CO2 through anaerobic metabolism (Awasthi et al., 2022; Robinson, 2002). Fungi like Aspergillus and Penicillium are known for their ability to produce enzymes such as amylases, proteases, and lipases, which can be utilized in the hydrolysis of complex organic compounds in dairy waste for further processing.
Algae: Algae have the potential to valorize dairy waste through the process of photosynthesis, where they utilize CO2 and nutrients from the waste stream to grow and produce biomass. Algal biomass can be harvested and processed into various products such as biofuels (biodiesel, bioethanol), high-value chemicals (pigments, antioxidants), and animal feed supplements (Awasthi et al., 2022).
Bio-electrochemical systems (BES; electrochemically active microbes): BES for dairy waste valorization involve the integration of biological and electrochemical processes to convert organic matter present in dairy waste into useful products such as electricity, hydrogen, or methane. The mechanism typically involves the utilization of microbial electrochemical reactions facilitated by specialized microorganisms, known as electroactive bacteria, which can transfer electrons to or from electrodes. In the case of dairy waste, organic compounds serve as substrates for microbial metabolism within the BES (Bhattacharya et al., 2023; Godbole et al., 2023). In an anodic chamber, organic matter undergoes oxidation by electroactive bacteria, releasing electrons that flow through an external circuit to a cathode. Concurrently, protons generated during oxidation migrate through an ion exchange membrane to the cathode, where they combine with electrons and possibly with other compounds (e.g., oxygen, nitrate) to form reduced products such as hydrogen gas or methane (Godbole et al., 2023; Nguyen et al., 2024). The overall process results in electrical current generation and valuable product production. This integrated approach holds promise for sustainable waste management and resource recovery in the dairy industry.
Dairy waste could be utilized as a substrate for the manufacturing of a variety of compounds. The compounds derived from dairy waste are useful in sustainable energy generation, the food industry, pharmaceutical industries, cosmetics, petroleum, and agriculture. Some common products include biofuels, biopolymers, biogas, bioactive compounds, biosurfactants, enzymes, etc. (Adesra et al., 2021; Capanoglu et al., 2022). The value-added dairy waste products are presented in Table 2.
| S/N | Product class | Valorized products | Microbial domains | Microbes/microbial strains | Ref. |
|---|---|---|---|---|---|
| 1 | Biofuel | i. Bio-hydrogen | Archaea | Methanobacterium sp. | Rosa et al. (2014) |
| ii. Bio-diesel | Algae | Chlorella protothecoides | Espinosa-Gonzalez et al. (2014) | ||
| Bacteria | Streptococcus thermophiles Lactobacillus delbrueckii subsp. Bulgaricus |
Vasiljevic and Jelen (2001) | |||
| iii. Biomethane | Yeasts |
Kluyveromyces lactis
Kluyveromyces marxianus |
Guimarães et al. (2010) | ||
| iv. Bioethanol |
Kluyveromyces marxianus
Kluyveromyces lactis |
Sampaio et al. (2020); Yamahata et al. (2020); You et al. (2017) |
|||
| Bacteria | Escherichia coli | Akbas et al. (2014); Sar et al. (2017a); Sar et al. (2017b); Sar et al. (2021) |
|||
| 2 | Biopolymer | i. Poly-3-hydroxybutyric acid (PHB) | Bacteria | Pseudomonas hydrogenovora | Koller et al. (2010) |
| ii. Polyhydroxyalkanoates (PHAs) |
Thermus thermophilus
Ralstonia eutropha Alcaligenes latus Aeromonas hydrophila Pseudomonas putida |
Pantazaki et al. (2009); Sudesh et al. (2000) |
|||
| iii. Poly (β-L-malic acid) (PMA) | Filamentous fungi | Aureobasidium pullulans | Xia et al. (2021) | ||
| 3 | Surfactants | i. Biosurfactant | Bacteria |
Lactococcus lactis
Streptococcus thermophiles |
Rodrigues et al. (2006) |
| 4 | Bio-active compounds | i. Lactobionic acid | Pseudomonas taetrolens | Goderska et al. (2014) | |
| ii. Nisin Z | Lactococcus lactis | Amiali et al. (1998) | |||
| iii. Plantaricin | Lactobacillus plantarum | Sharma et al. (2021); Zotta et al. (2020) |
|||
| iv. Pediocin | Pediococcus acidilactici | Guerra et al. (2005) | |||
| v. Enterocin AS-48 | Enterococcus faecalis | Ananou et al. (2008) | |||
| Yeasts | Kluyveromyces lactis | ||||
| Filamentous fungi | Aspergillus oryzae | ||||
| vi. Glactooligosaccharides (GOSs) | Bacteria |
Bacillus circulans
Lactobacillus reuteri Bifidobacterium bifidum Bifidobacterium infantis Lactobacillus acidophilus Lactobacillus pentosus Bifidobacterium longum |
Nath et al. (2015) | ||
| 5 | Biomass | i. Single-cell protein (SCP) | Yeasts |
Kluyveromyces marxianus
Kluyveromyces fragilis |
Schultz et al. (2006) |
| ii. Fungal biomass | Filamentous fungi | Aspergillus oryzae | Mahboubi et al. (2017a); Mahboubi et al. (2017b); Thunuguntla et al. (2018) |
||
| 6 | Enzymes | i. β-galactosidase | Filamentous fungi | Aureobasidium pullulans | Kaur et al. (2015) |
| Yeasts |
Kluyveromyces marxianus
Candida pseudotropicalis |
(Kaur et al. (2015) | |||
| ii. α-amylase | Bacteria | Serratia marcescens | Romero et al. (2001) | ||
| 7 | Hormones | i. Gibberellic acid | Filamentous fungi |
Fusarium moniliforme
Aspergillus niger |
Cihangir and Aksöza (1997) |
| 8 | Vitamins | Vitamin B12 | Bacteria | Propionibacterium shermanii | Sar et al. (2022) |
| 9 | Solvents | Biobutanol | Clostridium acetobutylicum | Foda et al. (2010) | |
| 10 | Organic acids | i. Propionic acid | Propionibacterium strains | Atasoy and Cetecioglu (2021); Atasoy et al. (2020) |
|
| ii. Citric acid | Filamentous fungi | Aspergillus niger | El-Holi and Al-Delaimy (2003) | ||
| iii. Lactic acid | Bacteria |
Lactobacillus casei
Lactococcus lactis |
Panesar et al. (2010); Prasad et al. (2014) |
||
| iv. Acetic acid | Yeasts | Kluyveromyces fragilis | Mostafa (2001) | ||
| v. Gluconic acid | Filamentous fungi | Aspergillus niger | Mukhopadhyay et al. (2005) | ||
| vi. Succinic acid | Bacteria |
Anaerobiospirillum succiniciproducens
Actinobacillus succinogenes |
Lee et al. (2000); Louasté and Eloutassi (2020) |
||
| vii. Pyruvic acid | Klebsiella oxytoca | Cao et al. (2020) |
Limitations and Future Perspectives
The successful implementation of waste valorization strategies is influenced not only by the processes involved but also by the upstream supply chain. This includes the collection and transportation of waste from production locations to the biorefinery. Little or no research is done in this regard. The types of seasonal waste, the amounts on hand for valorization, the associated costs, and emissions of transportation are essential considerations that require further investigation. Other issues that can obstruct the pervasive implementation of waste valorization include technological lock-in, unfavorable regulations, inadequate investment, and optimization based on local conditions. Therefore, comprehensive analyses of these factors are necessary to ensure the successful adoption of waste valorization strategies.
Another area that requires future study is understanding the relationships between dairy waste’s complex composition and its performance during biological conversion processes. Dairy waste including whey, sludge, and fats, contains a complex mixture of organic and inorganic components, making separation and processing challenging. Additionally, selecting the appropriate microorganisms for the microbial conversion process is challenging due to its heterogeneous nature. Future studies should focus on meticulously studying the composition of various dairy wastes and how they impact their valorization via biological conversion processes.
Although integrated processes have been discussed earlier as a way to close the circular loop of dairy waste valorization, the issue of energy recovery maximization still presents future challenges. Dairy waste contains significantly high moisture content that hinders its applicability with processes such as gasification and pyrolysis. However, integrating biological processes such as anaerobic digestion and thermochemical processes could present a way of mitigating the energy requirement. The combination of thermochemical and biological processes to maximize resource recovery and enhance process sustainability should also be the focus of future studies.
Several researchers have explored anaerobic digestion as a way of removing antibiotic resistance genes from dairy waste (Su et al., 2024). However, it is challenging to accurately predict the removal efficiency. Data-driven methods have been used for process optimization and prediction of biofuel yields for thermochemical and biological conversion processes (Okolie, 2024). The application of data-driven methods including ML methods for the optimization and prediction of product yield during dairy waste valorization should also be the focus of future studies.
Dairy waste also presents a great precursor for the production of value-added bioactive compounds and green chemicals (Sadh et al., 2018). These compounds are natural substances found in living organisms that affect biological processes, such as antioxidants, flavonoids, and probiotics, used in pharmaceuticals for disease prevention, functional foods for health benefits, and cosmetics for anti-aging properties (Sadh et al., 2018). Exploring genetically engineered microbes to enhance the production of bioactive compounds should also be the focus of future studies. While different technologies for dairy waste valorization have been discussed in this study, a comprehensive lifecycle and techno-economic analysis should be performed to compare the economic and environmental impact of each technology.
Conclusion
According to the ethics of a circular economy, waste-to-wealth strategies have been gaining traction due to their potential to create economic and environmental benefits. One of the most prominent waste-to-wealth approaches is waste valorization, which involves converting waste materials into useful products or energy sources. Through the process of waste valorization, harmful waste components can be transformed into valuable resources, mitigating their negative influences on human health, the economy, and the environment. As such, waste valorization represents a promising approach to promoting sustainability and circularity in industrial processes. However, the review identifies the challenges in dairy waste valorization to include the collection and transportation of waste from production locations to the biorefinery, technological lock-in, unfavorable regulations, inadequate investment, and optimization based on local conditions. A lack of understanding of the relationships between dairy waste’s complex composition and its performance during biological conversion processes has also been identified as a challenge. Other challenges include the difficulty associated with the selection of appropriate microorganisms for the microbial conversion process, energy recovery maximization, and accurate prediction of the removal efficiency. The authors therefore advocate among other things the application of data-driven methods including ML methods for the optimization and prediction of product yield during dairy waste valorization.













