Introduction
Contamination of fresh and dried meat by pathogens is a persistent challenge in the food industry. Fresh meat, at the point of slaughter and in meat processing, is a transmitting medium for viruses, such as hepatitis E, hepatitis A, and norovirus (Bae et al., 2015; Park et al., 2010). Liu et al. (2019) reported that protein deterioration and lipid oxidation during storage are potential consequences of bacterial contamination in dry-cured meat products. Meat product spoiling mechanisms are associated with biochemical reactions involving both external microbes and internal enzymes (Pedrós-Garrido et al., 2018). Gram-positive bacteria, such as Staphylococcus aureus and Brochothrix thermosphacta and gram-negative aerobic bacteria, such as Pseudomonas and Enterobacteriaceae, are common spoilage microorganisms. Escherichia coli and Listeria monocytogenes are also major food pathogens. E. coli O157:H7 has caused numerous foodborne outbreaks in a variety of foods, such as hamburger meat, apple cider, leafy greens, and poultry products (Ravishankar et al., 2009). In terms of the total cost of foodborne illness, L. monocytogenes is among the top five pathogens (Ha and Kang, 2014). Thus, inactivating microorganisms and viruses is essential for ensuring the safety of food (Kim et al., 2024; Kulawik et al., 2022).
Conventional thermal processing can negatively affect the sensory and nutritional qualities of food products; however, non-thermal processing is a preservation technique that has a minimal negative impact on the nutritional and quality properties of foods (Hong et al., 2008; Rosario et al., 2021). The use of ultraviolet (UV) light irradiation as an alternative and less expensive method of effectively reducing the number of microbes on food surfaces has attracted interest in food science and food industry (Ha et al., 2016; Usaga et al., 2017). In a number of studies, UV light irradiation has been used to control major food-borne pathogens, such as E. coli O157: H7, Salmonella enterica, and L. monocytogenes (Fan et al., 2021; Holland et al., 2020; Keklik, 2020; Yeh et al., 2018).
In general, studies have shown that applying UV light to meat, poultry, and fish products can preserve their nutritional value and quality (Liu et al., 2019; Mikš-Krajnik et al., 2017; Pedrós-Garrido et al., 2018). However, considering the probable modifications of photosensitive molecules, UV-C treatment has the potential to affect the physicochemical characteristics of meat products, and several undesirable changes may occur in nutritional attributes. Absorption of UV energy by the food material depends on the wavelength of the UV light and the structure and photosensitivity of the food molecules (Söbeli et al., 2021). Bintsis et al. (2000) reported that short-wavelength UV light (UV-C range: 200–280 nm) is the most harmful to cells among UV because it is much easily absorbed by DNA than longer-wavelength (UV-A range: 320–400 nm) or mid-wavelength (UV-B range: 280–320 nm) UV light. Moreover, factors that affect the design and performance of UV sterilizers include mechanical properties such as UV light intensity output, density, and irradiation dose. Among them, pathogen resistance varies depending on the UV intensity, which is a major factor in the efficiency of UV-C treatment (Rosario et al., 2021). Hayashi et al. (2021) reported that 265 nm is suitable for inactivating pathogens (E. coli, S. aureus, and Bacillus cereus) due to protein damage. In addition, Bowker et al. (2011) reported that UV inactivation of E. coli was more effective at 275 nm than at 255 nm.
Studies on inactivation utilizing UV-C irradiation have shown its efficacy in reducing bacterial counts on meat, with or without altering quality (Reichel et al., 2020; Söbeli et al., 2021). UV-C radiation cannot penetrate deeper tissue layers; hence, its effect is restricted to surface decontamination. The effectiveness of UV-C treatment at various wavelengths is influenced by the chemical composition of the food product; hence it is unclear to what extent this preservation technique reduces bacterial contamination of processed meat products (Guerrero-Beltrán and Barbosa-Cánovas, 2004; Sastry et al., 2000). The present study investigated the effect of the physicochemical properties and inactivation efficiency according to UV-C wavelength and intensity on the inactivation of E. coli O157:H7, L. monocytogenes, and hepatitis E virus (HEV) in prosciutto. Additionally determined was the impact of UV-C light on the quality characteristics of meat products.
Materials and Methods
OURHOME (Seoul, Korea) provided the prosciutto. Chung-Ang University (Seoul, Korea) provided the HEV. Before usage, the bacteria E. coli O157:H7 (NCCP 15739) and L. monocytogenes KCCM 40307 (Difco, Detroit, MI, USA) were diluted in sterile saline water. All the chemicals used for the analysis were purchased from a local supplier and were of reagent grade.
The steps described here were according to the instructions of the supplier (OURHOME), as illustrated in Fig. 1. In total, 42 packages (14×3) of prosciutto were subjected to UV-C analysis (400 mm×300 mm×200 mm, W×L×H; OURHOME). The UV-C treatment was performed using one pack at a time, and the wavelength was 265 and 275 nm (different inactivation effect on pathogens), intensity was 10 and 50 mW, and the exposure time was 0–900 s. From one batch (one pack containing 10 slices of prosciutto), five slices were randomly chosen for texture analysis and the remaining slices for chemical analysis.
A digital camera (α350, Sony, Tokyo, Japan) was used to obtain images of prosciutto samples, and the characteristics were observed. The color values were measured with a color reader (CR-10, Konica Minolta Sensing, Tokyo, Japan) using a white standard plate as a calibration: CIE L*, CIE a*, and CIE b* were 97.83, +0.43, and +1.98, respectively. The device was positioned directly on the prosciutto’s surface at various points. The total color difference (△E) was calculated via computation as follows:
Where △ CIE L⋆, △ CIE a⋆, and △ CIE b⋆ represent the change in each color after UV-C treatment. Two slices from each of the three batches totaled six slices for the color measurement (n=6, three batches×two replications).
A pH meter (S-220, Mettler Toledo, Zurich, Switzerland) was used to measure the pH of 5 g prosciutto samples after homogenizing for 60 s at 25°C with 45 mL of distilled water. For every sample, three readings were obtained. The pH meter was calibrated at 25°C using a standard buffer solution (pH 4, 7, and 10). This measurement was made with six slices in total, two slices from each 3-batch (n=6, three batches×two replications).
The heat-drying method (AOAC, 2012) was used to determine the water content of the samples. A dry oven (OF-105, Daihan Scientific, Wonju, Korea) was used to weigh and dry a sample of prosciutto weighing about 2 g for about 6 h, or until a constant weight was achieved. Water content has been determined as follows:
Where W1 and W2 are the initial and final weights of the sample, respectively. This measurement was made with six slices in total, two slices from each 3-batch (n=6, three batches×two replications).
With minor adjustments, the technique outlined by Choi et al. (2018) was used to evaluate the prosciutto samples’ capability to hold onto moisture. A centrifuge tube containing about 1 g of prosciutto sample was used, and the tube was centrifuged at 3,000×g for 10 min at 4°C. The water holding capacity (WHC) was calculated as:
Where W1 is the initial sample weight and W2 is the sample weight after centrifugation. This measurement was made twice per batch (n=6; three batches×two replicates).
The texture of the samples was determined using a texture analyzer (CT3, Brookfield, Ringgold, GA, USA). A circular plate probe (TA4/1000; 38.1 mm in diameter) was used to compress samples that were 1 cm by 1 cm twice, to 50% of their initial height. The compression was performed at a speed of 1 mm/s with a trigger load of 100 g. Using new batches of prosciutto (n=15, three batches×five replications), every step was repeated five times.
Prosciuttos were cut into thin slices and the morphology was observed using a scanning electron microscope (TM4000Plus, Hitachi, Tokyo, Japan) with backscattered electron detection at 15 kV and 500× magnification.
A minor modification to the procedure provided by Lee et al. (2021) allowed for the determination of secondary lipid oxidation from the 2-thiobarbituric acid reactive substances (TBARS) values. After homogenizing the samples (4 g) in 16 mL of distilled water, the samples eluted for 30 min. After the homogenates (20 mL) were filtered, 0.5 mL of the sample was combined with 4.5 mL of TBA solution—a mixture of 0.375% TBA reagent, 15% trichloroacetic acid, and 0.25 N hydrochloric acid—to measure the amount of TBARS present. The sample was then heated in a shaking water bath (MaXturdy 45, Daihan Scientific) for 15 min at 95°C. After the heated mixture was cooled for 30 min at 25°C, it was centrifuged for 10 min at 25°C at 3,000×g. A spectrophotometer (MultiskanTM GO UV/VIS, Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the absorbance of the supernatant at 532 nm. This measurement was made with six slices in total, two slices from each 3-batch (n=6, three batches×two replications).
With minor adjustments, the Conway microdiffusion method (Lee et al., 2021) was used to determine the volatile basic nitrogen (VBN) content. A total of six slices were used, two from each of the three batches (n=6, two batches×three replications). After homogenizing the sample (4 g) in 16 mL of distilled water, the sample was left to elute for 30 min. Whatman No. 1 filter paper (GE Healthcare Life Science, Sheffield, UK) was used to filter the homogenate (20 mL). The 1 mL of filtered sample was added to the outer section of the Conway dish along with 1 mL of 0.01 N H3BO3 and 100 μL of Conway solution. The inner portion of the Conway dish was filled with a mixture of 0.066% methyl red and 0.066% bromocresol green in aqueous ethanol. In addition, the outer portion of the dish received 1 mL of 50% K2CO3. Following a 2 h incubation period at 37°C, the Conway dish was titrated with 0.02 N H2SO4 until the Conway reagent turned red. The VBN values were determined using the following factors: the weight (g) of the sample (S), the dilution amount (C), the factor of H2SO4 (f), the titration volume of 0.02 N H2SO4 (mL; A), and the titration volume of the blank (mL; B).
Samples were prepared with minor modifications to the Lee et al. (2023) method in order to verify the effects of UV-C irradiation. The following were added to a 20-g sample taken from each of the 15 packages: 1 mL of a 50-fold diluted HEV stock [106 plaque-forming units (PFU)/mL], 100 μL of a colony-forming unit (CFU)/mL of E. coli O157:H7 stock, and 100 μL of a CFU/mL of L. monocytogenes stock. The mixture was then packed into sterile polyethylene pouches. The packages were vacuum-packed and exposed to UV-C radiation after drying.
To verify the effects of UV-C, the samples were processed following Lee et al. (2023) procedures. After being aseptically moved to a filtered stomacher bag (3M, St. Paul, MN, USA), each sample was homogenized for 5 min and diluted with an equal amount of saline water. The mixture and the supernatant were centrifuged at 4°C for 30 min at 10,000×g and 15 min at 8,000×g, respectively. Using the QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany) and the manufacturer’s instructions, RNA was extracted from the supernatant.
The 1.8 mL of sterile saline was used to suspend 200 mg of the sample, which was then serially diluted with sterile saline to assess the inactivation of E. coli and L. monocytogenes. The samples were then blended with a stomacher blender (BKST-04C, BioKonvision, Gwacheon, Korea) for 2 min while enclosed in bags. Following homogenization, the samples were divided into 20 mL of Oxford agar (Oxford Listeria agar, Oxoid, Basingstoke, UK) for L. monocytogenes strain KCCM 40307 and EC 3M (Petrifilm, 3M) for E. coli O157:H7 at 36°C for 24 h. For every experiment (n=3; 1 batch×3 replicates), microbiological parameters were estimated in triplicate.
To test for the main effects (different UV-C wavelengths, intensities, and time conditions), the packages were randomized. All data obtained in this study were analyzed using a linear mixed model. To evaluate the physicochemical and microbiological parameters, the main effects and their interactions were included as fixed effects and random terms of replications (batch). Analysis of variance (ANOVA) was conducted to determine the significance of the model using SPSS software (ver. 24.0, IBM, Chicago, IL, USA). When the main effects (wavelength, intensity, and irradiation time) were significant (p<0.05), Duncan’s multiple range test was performed as a post-hoc procedure.
Results and Discussion
Changes in the appearance, scanning electron microscopy, and color of the prosciutto under various conditions are shown in Fig. 2 and Table 1. For the appearance of samples, the structural and colorimetric modification by irradiation condition were not obvious (Fig. 2). Accordingly, the microstructure of samples after irradiation seemed not to be markedly changed (Fig. 3). CIE L* and CIE a* values of the control and treated samples were not significantly different (p>0.05). The CIE L* value of the control was 60.53, which varied with the treatments, ranging from 55.27 and 63.16. Generally, UV-C irradiation did not have a significant impact on the CIE L* values of prosciutto (p>0.05), except for 265/10 for 1 min. In CIE a*, the values decreased after rise with irradiation time. Short-term irradiation at 275 nm significantly increased the CIE a* values of the samples (p<0.05). At 275/10, the CIE a* value of the samples raised from 15.54 to 18.60 for the 1 min treatment, likewise those of the samples were over 19 with irradiation at 275/50 for 1 and 5 min. However, CIE a* of the 275 nm-treated samples were reduced with longer irradiation treatments, presenting no marked difference from the control (p>0.05). The CIE b* value of the control was the lowest, and irradiation increased the CIE b* of prosciutto (p<0.05), except at 265/10 for 1 min. However, it was hard to find the effect on CIE b* by wavelength, intensity, or irradiation time.
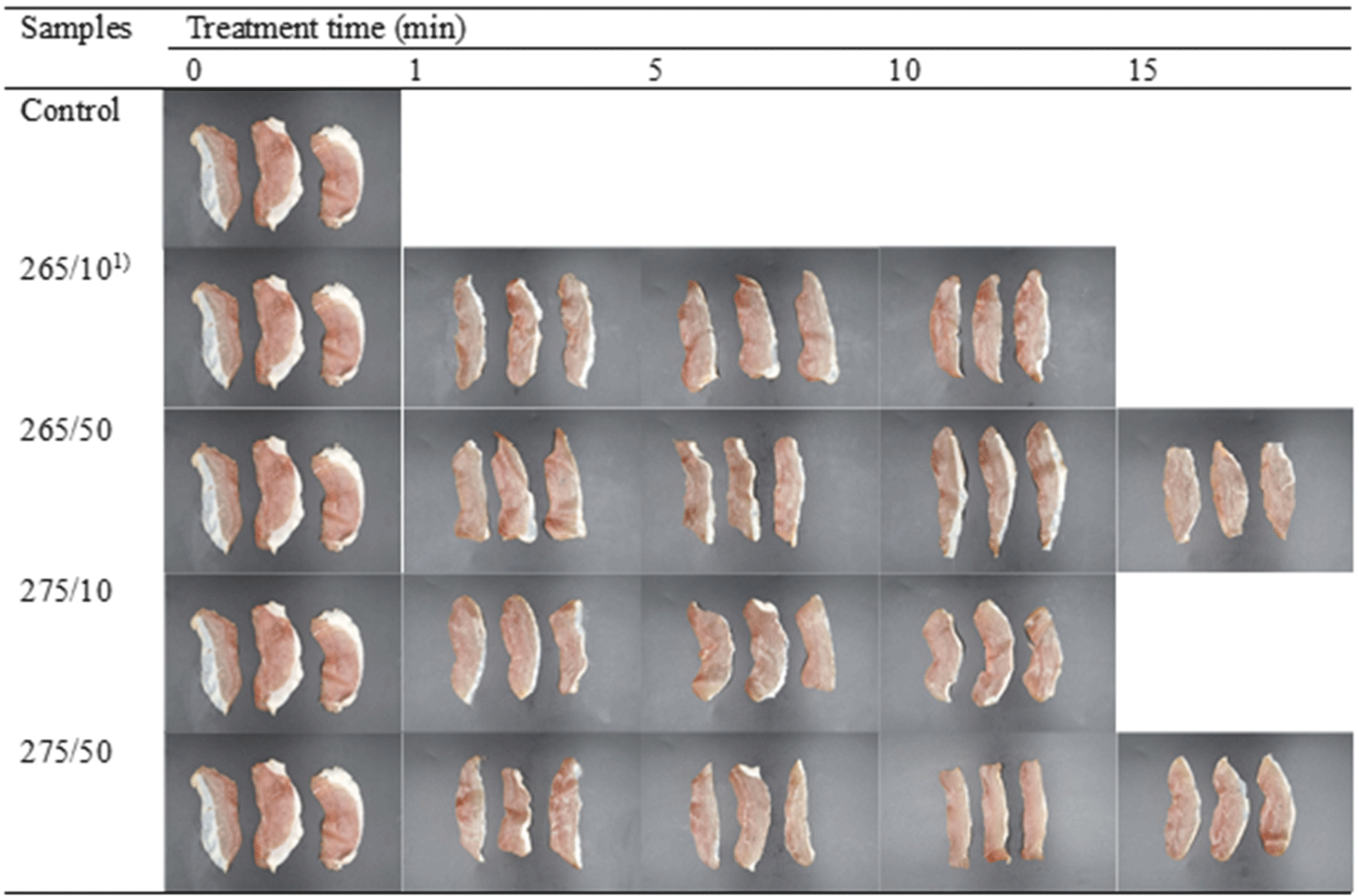
Color is one of the most crucial factors in defining the appearance of food products and influencing consumer preferences is color (Hong et al., 2012). UV-C irradiation can induce meat discoloration by forming metmyoglobin (Renerre, 1990). Park and Ha (2015) stated that chicken frankfurters with increasing stepwise UV-C (60, 3,600 mWs/cm2) showed a decrease in CIE L* value, indicating that higher UV-C doses resulted in lower CIE L* values and higher CIE a* and CIE b* values. Similarly, lower CIE L* values of chicken legs and breasts were reported after UV-C irradiation (82.56 mWs/cm2) for 1 min (Wallner-Pendleton et al., 1994), consistent with the results of the 1 min treatment in this study. Isohanni and Lyhs (2009) showed that activated oxygen lowers CIE L* value and increases the CIE a* and CIE b* values in meat after UV irradiation. The light-induced degradation of proteins and lipids (i.e., photooxidation) can change the color from red to brown (Söbeli et al., 2021). However, the CIE L* value rebounded and CIE a* value decreased with long-term irradiation. This was somewhat unclear, and thus, the total color differences among the samples were not significantly different (p>0.05), except for some samples, which remained below 10-unit in all samples. Jung et al. (2003) found that meat with a 10-unit increase in ΔE exhibits significant changes in appearance. Thus, the color change of prosciutto induced by UV-C irradiation was not perceptible to human eye in this study (Fig. 2). No significant differences were observed among the samples. This is consistent with the results of Park and Ha (2015), who reported no visual changes in meat color after UV-C irradiation.
Changes in the pH values of UV-C-treated and untreated prosciutto are shown in Fig. 4A. The pH values of all samples ranged from 5.75 to 5.94. There was a somewhat increasing tendency, although there was no distinct tendency caused by treatment because of the narrow distribution of values despite the statistical differences among samples. The pH of prosciutto depends on the rate of water loss, the increase in free amino acid content by proteolysis, and the drying environment (Drăghici et al., 2013; Petrovic et al., 2016). The pH of dry-cured ham was reported as approximately 5.8 (Alba et al., 2012; Bover-Cid et al., 2011), and UV-C irradiation did not significantly affect the pH of meat (Chun et al., 2010; Park and Ha, 2015; Söbeli et al., 2021), which were consistent with this result. In addition, Bintsis et al. (2000) reported no differences between foods treated and untreated with UV-C light. Monteiro et al. (2019) described that UV-C causes the production of reactive oxygen species, which subsequently cause the oxidation of water molecules. This process increases the ionic strength of the protein and exposes additional water-binding sites, thereby increasing its charge state. Though, it was regarded that the pH of samples was in the range of 5.75–5.94 regardless of UV-C doses and intensity, which might be due to the low moisture content of prosciutto.
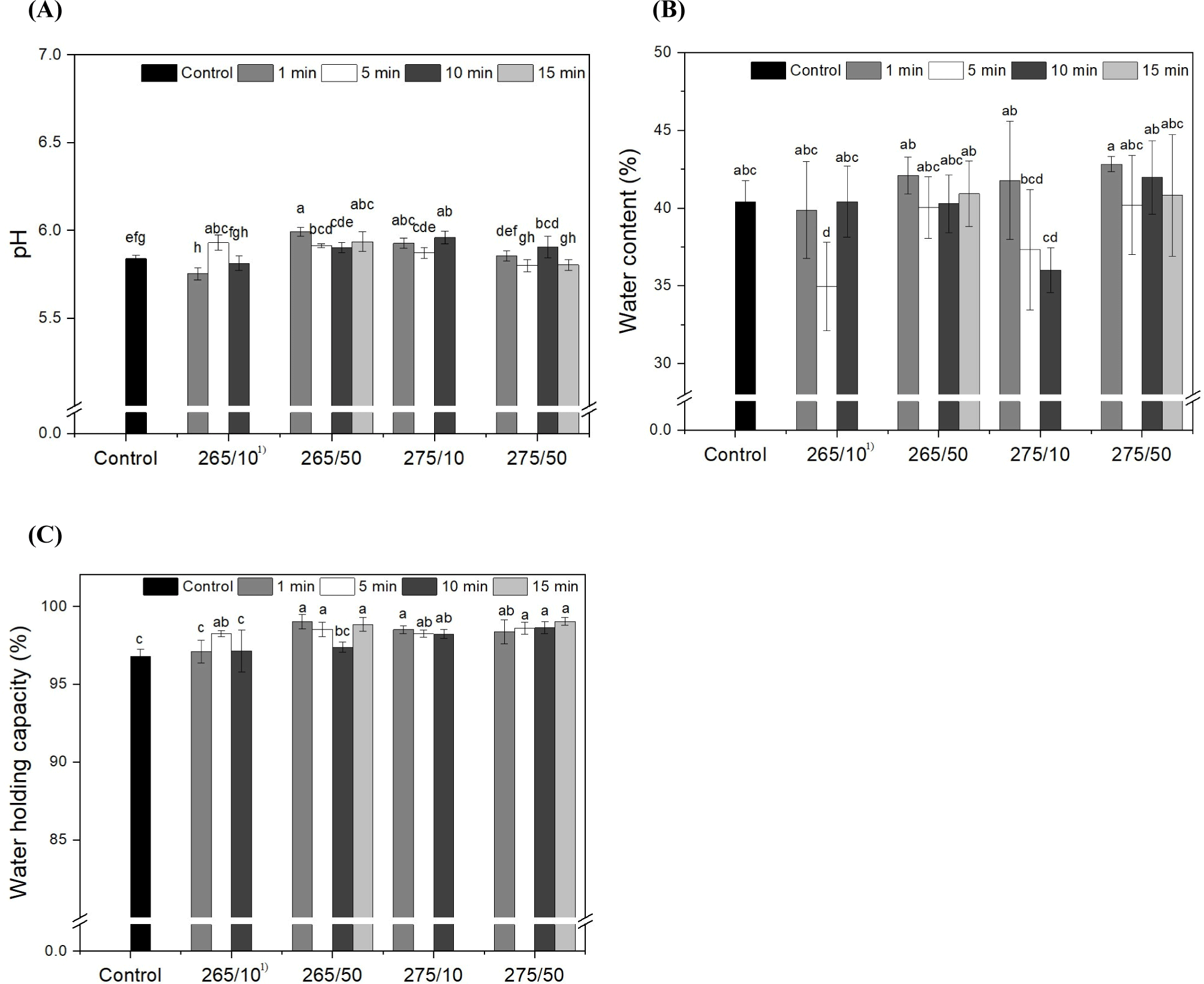
The water content and WHC of the prosciutto samples depending on the UV-C wavelength, intensity, and irradiation time, are presented in Figs. 4B and C. The water content of control was approximately 40%. After UV-C irradiation, except 265/10 for at 5 min, the water content of all samples did not change significantly (p>0.05). The WHC of the control was approximately 97%. After the irradiation, except for 265/10 for 1 and 10 min and 265/50 for 10 min, the WHC of all samples significantly increased to above 98% (p<0.05). Though, WHC of prosciutto was ranged into 97%–99% regardless of UV irradiation conditions.
The water content of prosciutto decreases from 60% to about 30% in one month during dry aging (Drăghici et al., 2013). Pleadin et al. (2017) reported that the water content of a household-manufactured prosciutto was 35%. Short-term treatment with no more than 15 min of irradiation had no effect on the water content of the prosciuttos. The WHC of meat is the capacity of muscle to retain moisture from external forces (Huff-Lonergan and Sosnicki, 2002). The increase in the WHC of prosciutto might be somewhat correlated with the pH rather than with irradiation. Arnau et al. (1998) reported that high-pH hams had higher WHC than low-pH hams. The pH trend was partially similar to that of the WHC results, except at 275/50. This can be induced by tissue aggregation, which contributes to the blocking of moisture loss. Wu et al. (2015) reported that the gel strength of gelatin exposed to UV irradiation increased owing to the UV-induced formation of new hydrogen bonds and the consequent aggregation of gelatin helices. As reported by Monteiro et al. (2019), during the entire storage period at 4°C for 15 d, the tilapia fillets treated with UV-C (103 mJ/cm2) displayed significantly higher WHC compared to untreated control groups, which may be because more water-binding sites were exposed after UV-C treatment. However, in this study, it was thought that UV-C irradiation did not markedly effect to water content and WHC of prosciutto.
Table 1 shows the hardness of the prosciutto after UV-C irradiation. The hardness of the control was the lowest (34.88 N) and UV-C irradiation significantly increased the hardness of the prosciutto (p<0.05). Excluding some samples, such as 265/10 for 1 min, 265/50 for 5 min, and 275/50 for 15 min, which were approximately 49 N, the UV-C treatment significantly increased the hardness of the prosciutto to over 50 N (p<0.05). However, the individual effect of wavelength and intensity on hardness of samples appeared to hard to find obviously. There was no significant difference in the hardness among the samples within the same irradiation time (p>0.05).
Textural parameters are crucial for determining quality, which affects consumer satisfaction (Hong et al., 2005). Esua et al. (2021) reported that tissue hardening was associated with myofibrillar protein accumulation and denaturation. However, numerous studies have reported that UV-C treatment does not affect the textural properties of chicken breast, poultry, or pork (Chun et al., 2010; Monteiro et al., 2021). Degala et al. (2018) noted an increase in the hardness of UV-C-treated goat meat, although the difference was not statistically significant (p>0.05) ascribing to tissue aggregation and structural changes, which leaded to the increase in WHC and hardness (Wu et al., 2015). In this study, any distinguishable modification in the morphology of prosciutto was not detected after irradiation (Fig. 3).
The effect of UV-C radiation on the TBARS value of prosciutto is shown in Fig. 5A. The TBARS level in the control group was 0.614 mg MDA/kg. Generally, the TBARS of samples increased with the wavelength and intensity. Especially in 275 nm, higher intensity induced higher TBARS level of samples (p<0.05). For wavelength, the tendency was roughly found that the TBARS of 275 nm-treated samples were higher than those of 265 nm-treated samples in same irradiation time. Thus, the 275/50 treatment resulted in significantly high TBARS within 5 min irradiated samples (p<0.05). After 10 min of irradiation, the samples treated with 50 mW showed significantly higher TBARS values than those treated with 10 mW (p<0.05). After 15 min of irradiation, the TBARS values were reduced and were not significantly different from the corresponding values in the control (p>0.05). Among the main effects (wavelength, intensity, and irradiation time), the intensity of UV-C had a marked effect on the TBARS of the prosciutto, although all values were less than 1.0 mg MDA/kg.
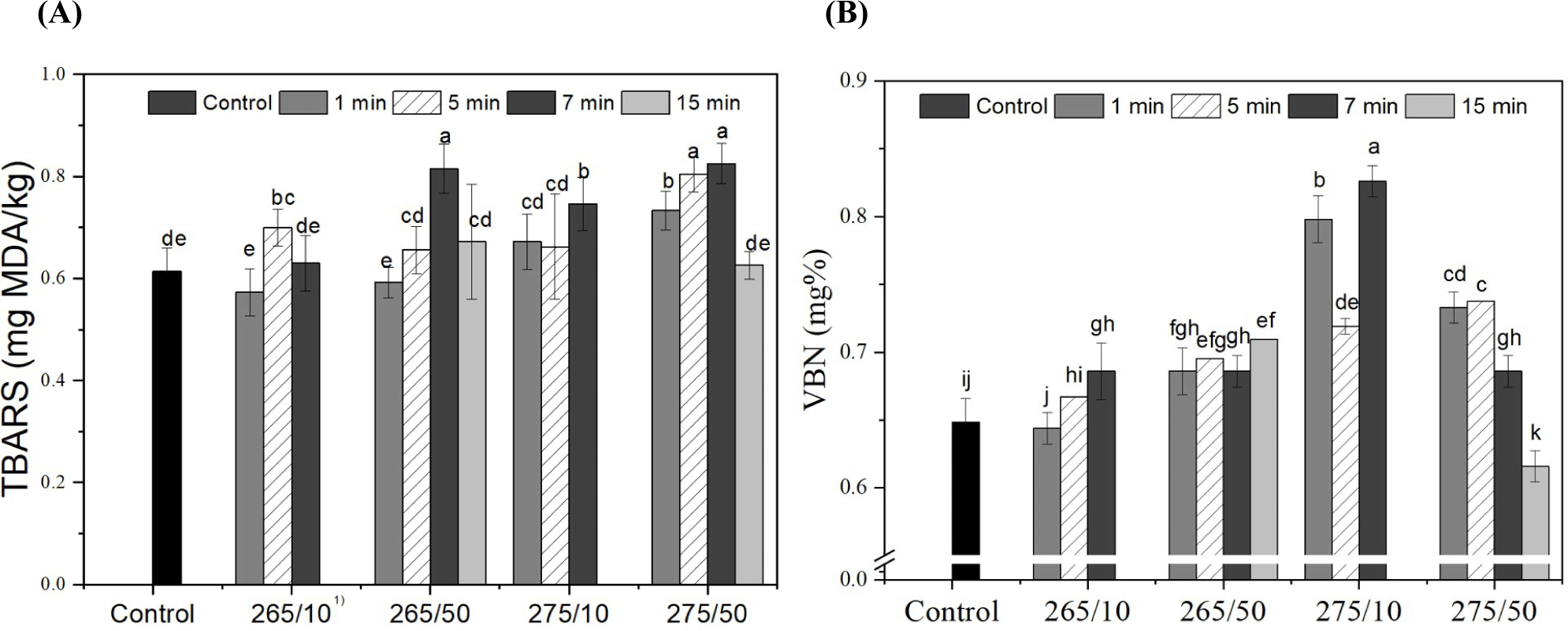
Lipid oxidation is a complex process and an important indicator of meat deterioration (Fan et al., 2021; Lee et al., 2021). UV irradiation can result in the production of reactive chemical species such as hydrogen peroxide, hydroxyl radicals, and superoxide anions (Fan et al., 2021). The higher intensity and longer irradiation time of UV-C could be associated with a pro-oxidant effect that produces peroxide radicals and accelerates lipid oxidation (Koutchma, 2019). The composition of fatty acids and the amount of fat in foods exposed to UV-C determine changes in TBARS values (Kim et al., 2011). Namiki (1990) reported that various factors of lipid oxidation initiation, such as peroxides, the presence of oxygen, heat, light irradiation, and enzymes, affect TBARS. Chun et al. (2010) reported that the TBARS value of chicken breasts gradually increased during storage, even at the same UV-C dose. Fan et al. (2021) reported an increase in TBARS in tuna fillets after UV irradiation. The TBARS increase was further observed in the UV-C treated goat meat, chicken breast, and tilapia fillet (Degala et al., 2018; Lázaro et al., 2020; Park and Ha, 2015), consistent with this study. Although, the level of TBARS in all samples was below 1.0 mg MDA/kg, the threshold of rancid odor of meat product (Kolsarıcı et al., 2010).
VBN of the prosciutto depending on UV-C irradiation is illustrated in Fig. 5B. The initial VBN was 0.65 mg% and UV-C irradiation significantly raised the VBN contents of prosciutto, with some exceptions (265/10 for 1 and 5 min, and 275/50 for 15 min; p<0.05). The VBN values of 275 nm-treated samples were higher than those of samples irradiated at 265 nm, except 50 mW for 10 and 15 min (p<0.05). However, there was no clear trend based on intensity and duration of irradiation. Further, all values were below 1 mg%.
VBN compounds consist of ammonia and amines produced by disintegrated proteins; thus, VBN is considered a freshness index for meat products (Lee et al., 2022). Monteiro et al. (2019) reported that UV-C radiation acts as a pro-oxidant. Fatty acid molecules reach electronically excited levels, absorb photons, and trigger dissociation reactions that generate free radicals. Thus, higher doses result in higher levels of excitation, and consequently, higher numbers of free radicals that enhance the oxidation of lipids and proteins (Canto et al., 2016; Koutchma, 2019). Oxidized lipids and proteins produce free radicals, which cause changes in the myofibrillar protein structure and expose hydrophobic amino acids, making them more susceptible to proteolytic enzyme action (Monteiro et al., 2019). According to Kim et al. (2019), the standard VBN value of fresh meat is below 20 mg%, and all samples were susceptible to UV-C irradiation, despite a significant increase in VBN. It was thought that UV-C could cause oxidative stress in the proteins and lipids of the prosciutto, although its impact on VBN was minor.
The total numbers of aerobic bacteria are shown in Table 2. In all samples, the total number of colonies was below the detection limit (1 Log CFU/g; Jo et al., 2020). Total aerobic bacteria ranged between 6.03 and 7.25 Log CFU/g, and there were no significant differences between the control and treated samples. Among the treated samples, the UV condition at 265/10 for 600 s showed the lowest value (p>0.05). The reduction rate was highest at irradiation times of 5 and 10 s at 10 and 50 W, respectively, regardless of the wavelength. Soro et al. (2021) reported that the application of UV light of various wavelengths to chickens significantly reduced the mean bacterial concentrations. A lower reduction compared to that of the control was also observed at specific treatment times; however, the difference was not significant. A similar trend was observed in the present study.
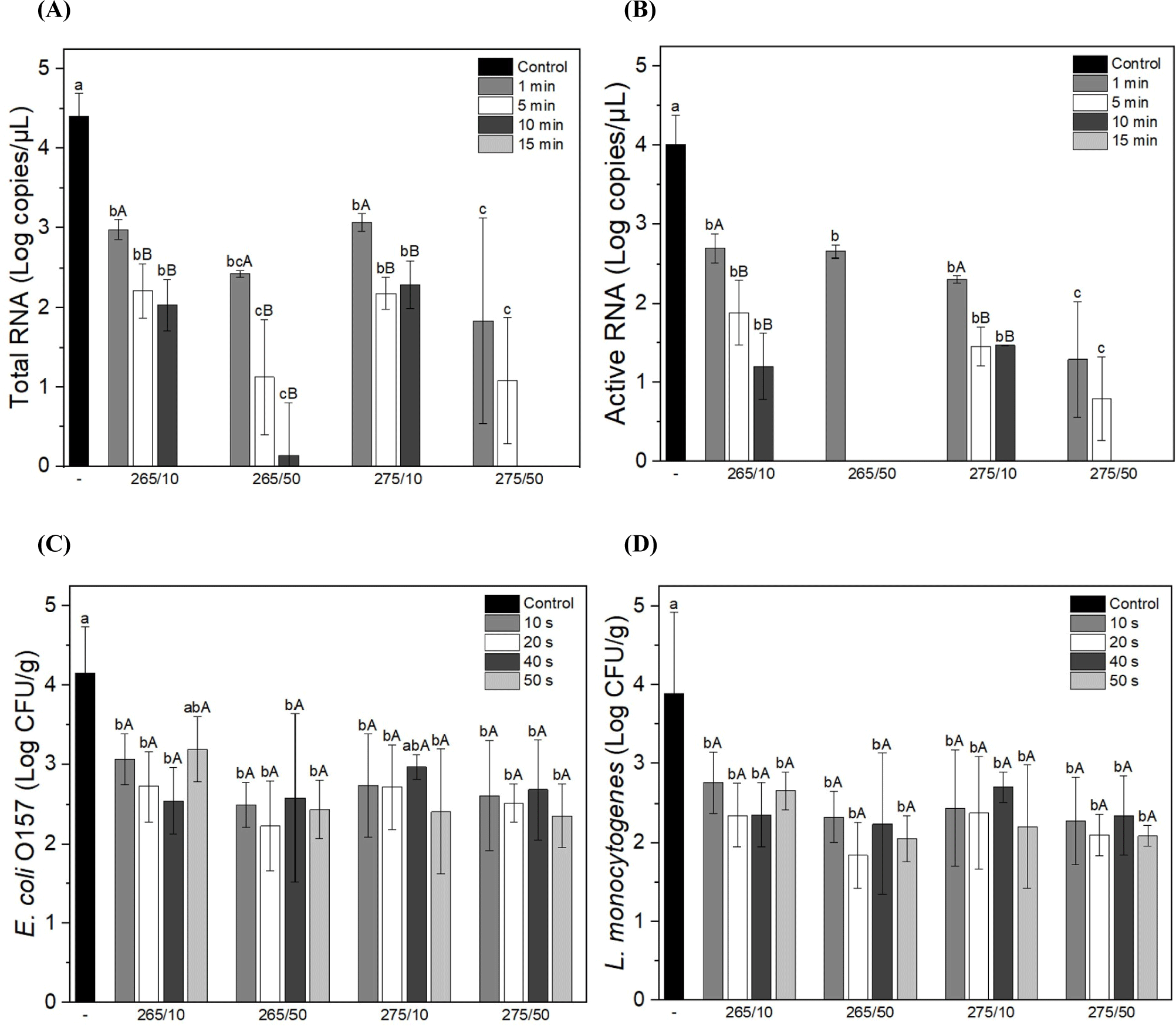
As shown in Figs. 6A and B, both the total and active HEV RNA were significantly reduced after UV-C treatment compared to the control, and the amount of RNA decreased with increasing exposure time in all samples. UV radiation produces photoproducts that cause structural DNA deformation and cell death (Mikš-Krajnik et al., 2017). Gómez-López et al. (2021) reported that UV radiation affects viral inactivation by inducing protein damage. However, another report stated that meat products are slightly less effective in virus inactivation owing to UV ray shielding by irregular surfaces (Gómez-López et al., 2007). This ccurrent study found that both E. coli and L. monocytogenes were significantly reduced after UV-C treatment compared with the control (Figs. 6C and D). Based on these results, UV-C light effectively and dose-dependently inactivated microorganisms in protoplasts. Similarly, Sommers et al. (2010) reported that sausages irradiated with UV-C light showed a reduction in L. monocytogenes, S. aureus, and Salmonella. Other factors, such as the species, strain, and composition of food, can also affect the effectiveness of UV-C radiation in reducing bacterial counts (Reichel et al., 2020; Sommers et al., 2010). Furthermore, Reichel et al. (2020) found that the surface texture was the most influential factor in decreasing bacterial counts when using UV-C light. The current study found that UV-C treatment effectively reduced viruses and pathogens by up to 4 Log; therefore, as further evidence, the number of microorganisms in meat products can be reduced by UV-C radiation. It should be emphasized that this decreasing effect may depend on the species or strain of the microbe.
Conclusion
UV-C irradiation successfully reduced in the load of microorganisms on the prosciutto without perceptible modification in appearance and microstructure. Further, the UV-C irradiation did not markedly affect to water content and WHC of prosciutto. Although, the TBARS and VBN of prosciutto was raised after irradiation, it was not exceeded the threshold for rancid deterioration. Specifically, a significant reduction in both E. coli and L. monocytogenes was observed in all UV-C-treated samples compared with the unirradiated sample. In particular, the reduction of HEV by UV-C irradiation was significant, and no active RNA was detected at 265/50 and 275/50 for more than 10 min. Photoproducts affect microbial inactivation by inducing protein damage, which leads to DNA modification and cell death. Briefly, these results indicate that UV-C above 265 nm at 50 mW for 10 min inactivated microorganisms without quality modification of the meat product. These findings support further application of UV-C irradiation in meat processing.













