Introduction
Seasoning of meat is a possible solution to enhance the color stability, minimize lipid oxidation and improve microbial safety of meat products. The seasoned meat products are more susceptible to spoilage of microorganism (Rysman et al., 2016). These products are usually marketed at refrigerated temperature (2°C−5°C) in order to increase shelf life (Radha Krishnan et al., 2015). The spoilage due to the lipid oxidation has deleterious consequences on the quality of fresh meat and other meat products resulting in massive economic losses (Shahidi and Zhong, 2010). While microbial activities in the food products may also destroy the quality of meat by the development of undesirable responses involving the worsening of odour, color and textural properties of food products (Lucera et al., 2012).
The spoilage factors in meat products sometimes produce toxic materials which are hazardous for human health (Jiang and Xiong, 2016). Therefore a vast amount of antioxidants, which have been chemically synthesized, are added to the meat products, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). However, use of potentially carcinogenic substances is not permitted in some countries (Falowo et al., 2014). Therefore, meat industry is largely applying antioxidants from plant sources as safer alternatives and preservatives (Devatkal and Naveena, 2010).
A number of plant materials have been used directly or indirectly, as seasoning materials for the antimicrobial purpose, to improve the meat quality (Appendini and Hotchkiss, 2002). The natural preservatives used for the meat producers include cinnamon, clove, rosemary, basil, thyme, oregano, lemon leaf, ginger, basilica, balm, coriander and many of them are generally recognized as safe (GRAS) in food industry (Alfonzo et al., 2017; Jiang and Xiong, 2016; Khaleque et al., 2016). Chinese cinnamon (Cinnamomum cassia) is usually add to the deserts, drinks or bakery products, majorly in South Asian and Central Asian regions, and considered as a vital spice in many meat products. Cinnamon is commonly used in meat and fast food products for culinary purpose, and has been usually described as a powerful antioxidant and antibacterial agent in the seasoned meat and fish products (Al Sahlany, 2017; Ozogul et al., 2017). Previous study has been confirmed that cinnamon has effective free radical scavenging activity due to the presence of bioactive compounds (Radha Krishnan at al., 2014). Cinnamaldehyde is the major constituent responsible for it high antioxidant activity (Dudonne et al., 2009) and the ability of cinnamon to preserve meat quality has been tested in beef burger, grass crabs and inactivate Listeria monocytogens in ground beef and chicken meatballs (Ghabraie et al., 2016; Huang et al., 2017; Khaleque et al., 2016).
The effects of adding Chinese cinnamon powder directly into the ground lamb meat to maintain meat quality has not been tested previously. Therefore the aim of this study was to examine the effectiveness of different concentrations of Chinese cinnamon powder on the ground lamb meat quality. For this purpose, concentrations of 0.5%, 1.5%, and 2.5% Chinese cinnamon powder were added to the ground lamb meat and total viable counts (TVC), pH, lipid oxidation and color attributes were evaluated. Furthermore, the antioxidant activity of Chinese cinnamon aqueous extract was also measured. This study may provide potential implications in substituting synthetic antioxidants with natural ones.
Materials and Methods
Dried bark of Chinese cinnamon was obtained from a local supermarket. Chinese cinnamon bark was divided into small pieces and grounded to powder by a high performance kitchen grinder (High Speed Universal Grinder, Tianjin, China). The powder was sieved with the help of sieve (1.651 mm, ASTM No. 10), and packed into 100 g packs and then stored in high-density polyethylene bags at 25°C until its further usage. Exactly 50 g powder was refluxed by using 450 mL distilled water for 5 h in enclosed flasks with constant shaking at 180×g by following the method from Sivarajan et al. (2017) to obtain a 10% w/v water extract. Then the extract was cooled and filtered twice using Whatman filter paper and used for performing the DPPH antioxidant assay.
The DPPH-radical-scavenging activity of aqueous extracts was assessed by following the protocol of Elahi and Mu (2017). Chinese cinnamon and BHT at concentrations of (20, 40, 60 mg/mL) were prepared by addition of 2 mL of freshly prepared DPPH solution (0.1 mM in 95% methanol). The solution was vortexes using a mixer and incubated in dark at 27°C for 40 min. After that, each sample absorbance was checked at 516 nm using a UV spectrophotometer (Shimadzu UV-1800 Spectrophotometer, Kyoto, Japan) at room temperature. The radical-scavenging activity of samples were calculated in to percentage by following equation.
where “AE” showed the absorbance of solution at 516 nm after mixing the 1 mL of all samples with 2 mL of 0.1 mM DPPH solutions and 30 min incubation at room temperature, while “AD” showed the absorbance of 2 mL 0.1 mM DPPH solutions mixed with 1 mL Milli-Q water.
Fresh lamb meat (Oyster muscles) was purchased from a local market at 24 h post-mortem and was placed in insulated polystyrene ice boxes and transferred to the laboratory within 1 h. The muscles were trimmed to remove connective tissues under hygienic conditions and minced using a mincer machine (8 mm plates). The minced lamb meat samples were assigned to the following four treatments: control (without any additive), 0.5%, 1.5%, and 2.5% of Chinese cinnamon powder applied on the 100 g ground lamb meat. Immediately after adding the Chinese cinnamon powder, samples were thoroughly hand-mixed using a bowl mixer and 100 g ground lamb meat (round shape and 1.5 cm thick) was prepared for each treatment with three replicates. The lamb meat samples were vacuum packaged (VP), properly labelled and stored at 4°C for 16 days. The vacuum packaged samples were sealed in polyethylene bags (20/70 mm) (Vacioplast, Salamanca, Spain) with an oxygen permeability lower than 40 cm3/(m2 day atm). After that, the samples were collected at 0 (approximately 24 h post mortem), 4, 8, 12 and 16 days of storage. At the time of sampling, 10 g sample was collected immediately under aseptic conditions for microbiological analysis. While the meat color was determined after blooming for 30 min at 4°C and then the remaining samples were frozen at −80°C priors to thiobarbituric acid reactive substances (TBARS) analysis. The above experiment was carried out in triplicates.
The pH value of ground lamb was determined by pH meter (Testo 205 pH meter, Lenzkirch, Germany). Before measurement, pH meter was calibrated by buffers of different pH concentrations i.e. 4.00 and 7.00 at 25°C. The glass rod of pH meter was inserted directly into the ground meat sample. Each time, four readings were recorded from different locations and averaged.
The meat surface color was recorded using Minolta spectrophotometer (CM-600d, Konica Minolta Sensing Inc., Osaka, Japan) with 8 mm diameter measuring aperture size Illuminant D65, 10° standard observer and CIE L*, a*, b* color score. Four measurements were recorded throughout the surface of selected samples (Li et al., 2017). The color of meat samples at 0 days was taken after collecting the fresh-cut surface from the local market. At each time point the vacuumed packed samples were opened and the surface color of ground lamb meat was measured at 0, 4, 8, 12, and 16 days after 30 min of blooming at 4°C.
Lipid oxidation analysis was conducted with minor modification by following the method described by Belles et al. (2017). Briefly, 20 mL of 10% tricloracetic acid (VWR) was mixed with 10 g of meat and homogenized at 724×g by using ultraturrax for 90 s (T-10 basic, IKA-Werke, Staufen, Germany). Then the samples were centrifuged for 30 min at 10°C at 2,897×g (High-Speed Refrigerated Centrifuge, CR 21N, Hitachi, Tokyo, Japan). The supernatant was filtered by using filter paper and 2 mL of the filtrate was added with the equal quantity of TBA 20 mM (Sigma-Aldrich, St. Louis, MO, USA). After that, the mixture was vortexed and incubated in a water-bath at 97°C for 20 min. At the end, samples were cooled under tap water at ambient temperature of 15°C and absorbance was measured at 532 nm by using spectrophotometer (Shimadzu UV-1800 Spectrophotometer, Kyoto, Japan). The TBARS, mainly malondialdehyde (MDA), was calculated from a standard curve of 1,1,3,3-tetraethoxypropane (TEP; Sigma-Aldrich), the lipid oxidation was expressed as the average of three replicates per sample in mg malondialdehyde/kg meat.
TVC were inspected based on standard plate count method by the method of Zhang et al. (2016). In brief, 10 g sample was taken aseptically from vacuumed bags and homogenized in 90 mL of sterile physiological saline in stomacher bags for 1 minute. For microbial enumeration, suitable serial dilutions were prepared using the same diluent, by following the protocol of International Organization for Standardization’s (ISO, 2003). Then the deriving suspension was serially diluted (1:10) in sterile physiological saline water and 1 mL samples of appropriate dilutions were poured into the petridishes, containing 15–20 mL of plates count agar (PCA). The number of bacterial colonies on the plates were enumerated after incubation for 48 h at 37°C and expressed as Log CFU (colony forming units)/g meat.
The experiment was designed with Chinese cinnamon treatments and storage times as fixed factors and replicates as random factor. General linear model (GLM) was used to express the significance of differences (p<0.05) between means. Statistical analysis of data was performed using the IBM statistical package for social sciences (SPSS) Statistics 22 software (SPSS Inc., Chicago, IL, USA). Duncan multiple range test was applied to determine the significant difference (p<0.05). The data were expressed as the mean±SD. Experiments were replicated three times and all parametric measurements were carried out in duplicate.
Results and Discussion
The DPPH radical scavenging activity of Chinese cinnamon aqueous extract at the concentrations of 20, 40, and 60 mg/mL were considerably lower than the pure antioxidant BHT as shown in Fig. 1 (p<0.05). The Chinese cinnamon aqueous extract showed strong antioxidant activity in all concentrations but the highest antioxidant activity was seen in 60 mg/mL indicating the highest radical scavenging activity (p<0.05).
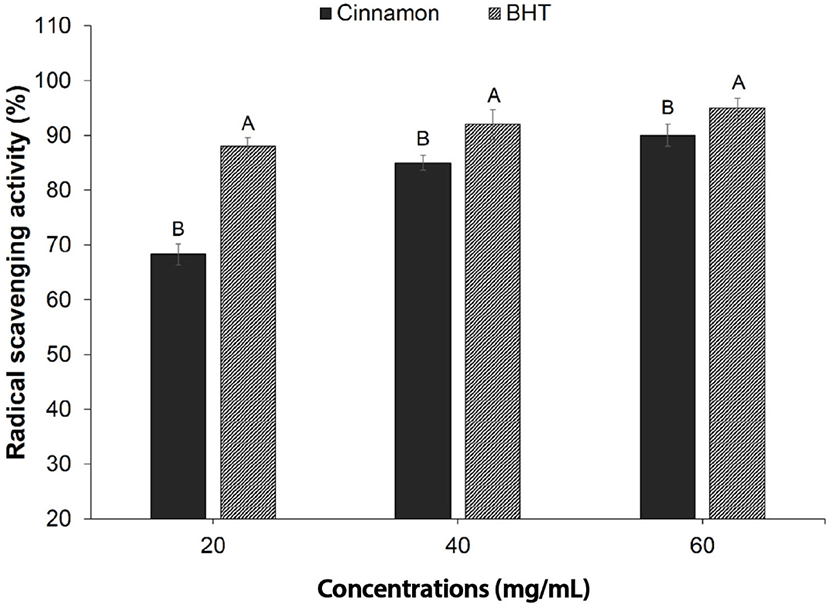
Previously reported studies have confirmed that cinnamon is distinguished by its effective radical scavenging activity due to bioactive substances (Radha Krishnan et al., 2014). The higher antioxidant activity in cinnamon might be due to the presence of significant amount of phenolic antioxidants and flavonoids compound (Jayaprakasha et al., 2007). Kuspradini et al. (2016) reported that cinnamon aqueous extract as well as cinnamon oil, have shown considerable antioxidant activity. Radha Krishnan et al. (2015) claimed that cinnamaldehyde was the responsible compound in the cinnamon for its high antioxidant activity.
The results of adding Chinese cinnamon powder on the pH of ground lamb meat stored at 4°C were presented in Table 1. The pH of control had a significant increased from day 12 to 16 while that of all other treatments samples remained the same during storage. The pH for all samples was same at day 0, while at day 4 the pH value of samples with 1.5% Chinese cinnamon powder was significantly lower (p<0.05) that of the control group and the other treatment groups. The pH values of samples from the control group and the 0.5% treated group had no significant difference with each other on day 8, whereas the stored samples with concentrations of 1.5% and 2.5% Chinese cinnamon powder showed a significant difference (p<0.05) compared with control group. With the increase of storage time, the samples with 2.5% concentration showed lower pH values at day 12 and 16 than control and other treated samples (p<0.05). Overall, the control group had a higher pH (p<0.05) at day 12 and 16 than that of other treatments during the storage.
The increment in the pH of sample stored under control group during the storage might be due to the generation of some basic compounds and ammonia caused by proteolysis resulting from the growth of microorganism (Chaijan et al., 2005; Masniyom et al., 2002). Radha Krishnan et al. (2014) and Brilliana et al. (2017) reported that during refrigerated storage, lower pH was noted in the raw beef meat samples treated with cinnamon oil compared to non-additive control. In the present study, the lower pH in the treatment samples might be due to the strong activity of bioactive compounds from the Chinese cinnamon powder.
The L*, a*, and b* of Chinese cinnamon powder treated and untreated ground lamb meat samples stored at 4°C were presented in Table 2. L* (lightness) value of samples from control group was significantly (p<0.05) higher throughout the whole storage time compared with other treatments. Among all Chinese cinnamon treated samples, the lower L* values were noted in samples with 2.5% concentration treatment in all storage days except for day 8 (p<0.05). The addition of Chinese cinnamon powder directly into the ground lamb might decrease the lightness of treated samples. The a* (redness) value of ground lamb was also affected by the addition of Chinese cinnamon powder during storage as shown in Table 2. The a* value of the control group showed a decreasing trend throughout the storage as compared to all the experiment groups. The initial a* values for all stored samples at 4°C were significantly different from each other (p<0.05). The a* values of control group and 0.5% treatment group were significantly higher at day 4 than those of samples with 1.5% and 2.5% treatments. At day 8 the a* value of control samples was considerably higher (p<0.05) than treated samples with 0.5% and 2.5% of concentration, while no significant difference was noted in samples from 1.5% treatment. As the days proceeded, at day 12 there were no significant differences between control group and all other Chinese cinnamon treated samples. The a* value for samples stored under control was lower than that of 1.5% treated samples at day 16, while no significant difference was found between samples with 0.5% and 2.5% of Chinese cinnamon powder (p<0.05).
The b* (yellowness) value for control group was higher at the initial day than that of the 1.5% and 2.5% treated groups (p<0.05), while no significant difference was noted in stored samples for 1.5% treatment. Whereas, on day 4 and 8 the only significant difference was noticed between control and 2.5% treatment groups than the samples with 0.5% and 1.5% treatment. The b* values for all stored samples included control and other Chinese cinnamon treated samples had no significant difference (p<0.05) after day 12 to 16 during storage.
It was proved that the meat discoloration was directly related with the storage length, and a* had a negative correlation with storage time (Terns et al., 2011), which might be possibly associated with an increase in TBARS (Grimsrud et al., 2008). Previous results confirmed that the addition of natural antioxidants might slow down the formation of metmyoglobin, ultimately delaying the deterioration of red color (Belles et al., 2017; Xia et al., 2009). Keokamnerd et al. (2008) observed that a* value was reduced in the minced chicken after 12 days of storage. The lessening in the intensity of redness values during storage was probably due to the relationship between lipid oxidation and color oxidation in the meat (Lynch and Faustman, 2000). In the current study, the fluctuations in the a* values were observed in all samples during the storage. The results were in accordance with the previous findings (Ozunlu et al., 2018; Zhang et al., 2016). The variation in the a* values might be due the MetMb% formation which can lead to the discoloration of the fresh meat (Krala, 2001).
The oxidative stability of control group and experiment groups was evaluated throughout the storage by determining the TBARS as shown in Fig. 2. The TBARS value was continuously increasing in the control group during the storage duration (p<0.05). Whereas, the TBARS values of Chinese cinnamon treated samples retarded during the storage intervals. Compared with experiment groups, the TBARS of control group was notably (p<0.05) higher during day 4 to 16. However, among all treatments, the samples with 2.5% treatment exhibited the lower TBARS values after day 8 to 16 (p<0.05). The TBARS value for the control was significantly (p<0.05) higher at day 16 than that of Chinese cinnamon treated samples. The results suggested that Chinese cinnamon powder was effective against the TBARS formation in the ground lamb during storage at 4°C.
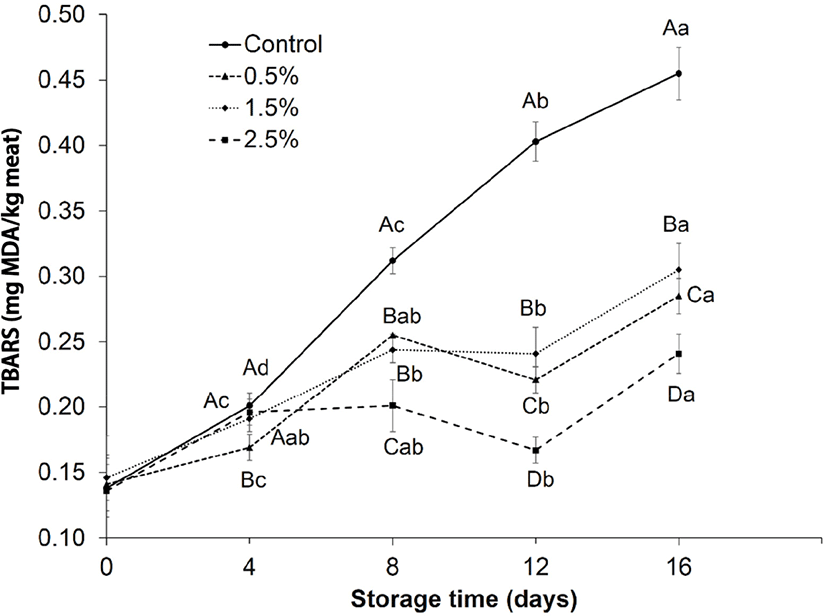
The cinnamon contained active compounds, which can lead to antioxidant and antibacterial actions in meat (Madsen and Bertelsen, 1995). Previous studies reported that the rapid increase in oxidation of control samples in the rainbow trout was due to non-availability of anti-oxidants (Shadman et al., 2017). The findings of present study agreed with the results of Shaltout et al. (2017), who also observed that the addition of cinnamon oil in beef delayed lipid oxidation during storage. Therefore, the reduction of TBARS in Chinese cinnamon treated samples may be caused by the presence of antioxidant compounds like cinnamaldehyde, eugenol and cinnamic acid (Dudonne et al., 2009). These antioxidants compounds may be useful against free radical damage (Dragland et al., 2003).
The effect of Chinese cinnamon powder on the TVC of ground lamb meat stored at 4°C for 16 days was presented in Fig. 3. The TVC of the control group increased immediately and rapidly than other samples treated by Chinese cinnamon powder. At initial day, there was no significant difference between the samples of the control and other treated samples. The TVC in control significantly increased (p<0.05) with the increment of time points at day 4 and crossed the limit of 7 log CFU/g at 16 days, while the treated samples retarded the growth of TVC. During the whole period of storage, the samples with 1.5% treatment showed significantly (p<0.05) lower TVC values at days 8 and 16, and the lowest enumeration was counted at day 8. From the obtained results, it was proved that TVC in the ground lamb meat may be inhibited by the addition of Chinese cinnamon powder (p<0.05).
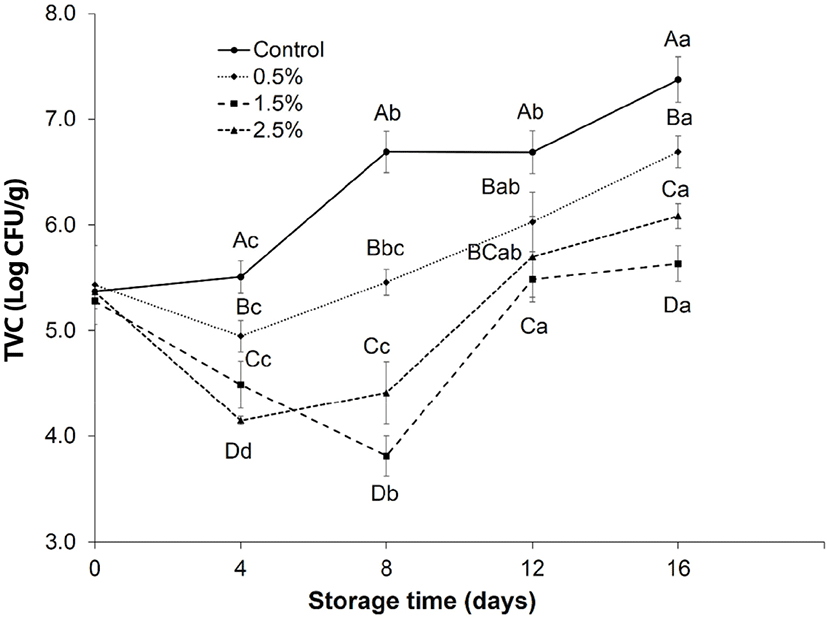
It has been reported that cinnamon inhibited microbes by several ways, such as rupturing of cell wall by the action antioxidant compounds, disordering the cytoplasmic membrane, cellular components disturbance by leakage, changed fatty acid and phospholipid constituents, affecting the DNA and RNA formation and destroying protein translocation (Bajpai et al., 2013). Comparable results were obtained by Gutierrez et al. (2008), where it was reported that the addition of cinnamon oil was more effective in decreasing the microbial counts in the food ingredients. In another study, cinnamon bark has been proved as a potential source against all pathogenic and spoilage bacteria (Ghabraie et al., 2016). Shaltout et al. (2017) observed that the incorporation of cinnamon oil was more efficient in maintaining meat quality. The reduction in the TVC during storage might be due to the presence of bioactive compounds present in the Chinese cinnamon powder.
Conclusions
In conclusion, Chinese cinnamon powder could maintain the quality of ground lamb meat by reducing the TVC and TBARS. The L* and a* values in the ground lamb meat can be affected during the storage by adding the Chinese cinnamon powder with a concentration higher than 0.5%. The results of the present study suggested that Chinese cinnamon powder at a level of 0.5% had the potential to maintain the ground lamb meat quality during storage. So it could be proposed as a natural alternative of synthetic additives to maintain the meat quality.













