Introduction
Cultured meat, also called in vitro meat or laboratory-cultured meat, is an edible tissue produced by the isolation, proliferation, and differentiation of muscle satellite cells (MSCs) obtained from a small amount of livestock tissue (Lee et al., 2021). The production of livestock products based on stem cell and tissue culture technologies is seen as a future technology and an emerging industry that is not only resource-efficient but can effectively address environmental degradation and the uncertainties associated with food security in the face of a growing global population and dwindling natural resources (Risner et al., 2023). Several countries around the world have implemented or taken steps to create policies to categorize cultured meat as cellular agriculture (Soice and Johnston, 2021).
A report by GlobeNewswire (2023) ascertained that the estimated value of the global cultured meat market was USD 182 million in 2022 and will continue to grow, with a projected CAGR of 23.2%. Currently, the only marketable cultured meats that have been officially certified as safe by the United States Food and Drug Administration (FDA) are cell-cultured chicken from Upside Foods and GOOD Meat.
Following this official approval, several companies worldwide are seeking permission to sell cultured meat. In July 2023, an Israel-based company, Aleph Farms, submitted a regulatory approval application to the Swiss Federal Office for Food Safety and Veterinary Medicine (Aleph Farms, 2023). Subsequently, in January 2024, Israel’s Ministry of Health (MoH) approved the sale of cultured beef from Aleph Farms, making it the third country to offer cultured meat for sale and the first approval for a bovine species (Aleph Farms, 2024). In October 2023, CellMEAT requested the Ministry of Food and Drug Safety (MFDS) of the Republic of Korea certification for Dokdo shrimp (Lebbeus groenlandicus) cell culture as a temporary food ingredient (CellMEAT, 2023). In December 2023, Food Standards Australia New Zealand (FSANZ) announced new amendments to an application received from Vow seeking approval of cultured quail (FSANZ, 2023). Likewise, research is underway around the world to produce cultured beef, pork, lamb, turkey, foie gras, and various types of seafood (oysters, lobster, shrimp, salmon, and tuna) using cell culture technology, and the development of various materials and equipment for cultured meat production, including adipocytes, supports, microcarriers, growth factors, and bioreactors, is gaining traction.
Despite expectations, the full-scale industrialization of cultured meat has not yet been achieved, and the timing of the industrialization of cultured meat remains unclear. In addition, the terminology for cultured meat has not yet been standardized. The Food and Agriculture Organization (FAO) and the World Health Organization (WHO) use the term ‘cell-based food,’ the United States Department of Agriculture-Food Safety and Inspection Service (USDA-FSIS) uses ‘cell-cultured meat,’ and the U.S. Food and Drug Administration (FDA) uses ‘cultured animal cell material’ (e.g., cultured Gallus gallus cell material; FAO and WHO, 2023; FDA, 2023a; USDA and FSIS, 2023).
Therefore, this review analyzes the main issues related to the industrialization of cultured meat, as well as research reports and media reports on the development of cultured meat to date, with the aim to present the current technology, industrialization level, and prospects of cultured meat.
Cultured Meat and Food Safety
Cultured meat production facilities are considered to be safer than conventional meat production facilities against foodborne pathogens, such as Salmonella, Campylobacter, Escherichia coli, yeasts, molds, and parasites because they are designed with enclosed structures that can control the entry of external substances (Chriki and Hocquette, 2020). However, potential threats from the cultured meat production process cannot be completely ruled out. Among the anticipated food safety concerns are contamination with microorganisms and prion proteins that may occur during the cell culture phase, residues of antibiotics and cell freezing agents, the safety of cell lines (genetic manipulation and excessive passage culture), exogenous recombinant growth factors, unknown allergens, and the safety of support materials (Broucke et al., 2023; Ong et al., 2021).
Furthermore, it is crucial to adhere to the guidelines of food authorities, such as the FDA, when using a scaffold for the production of cultured meat. This includes following regulations regarding the use of materials, solvents, cross-linking agents, inedible substances, toxic compounds, allergens, and other related factors (Levi et al., 2022). However, challenges remain in the commercialization of scaffolds due to the need to establish safety evaluation and approval standards for solvents or cross-linking agents used in scaffold polymerization, potential decomposition by-products of biodegradable scaffolds, physicochemical modifications of synthetic polymer scaffolds, and recombinant proteins that improve cell attachment efficiency (Bomkamp et al., 2022).
In response to these concerns, several countries, such as those in Australasia and the European Union (EU), Korea, Singapore, the United Kingdom, and the United States, have taken steps toward establishing regulations and classification guidelines for cell-based foods or temporarily allowing them as food ingredients (EU, 2021; FDA, 2019; FDA, 2023b; FSA, 2023; FSANZ, 2023; MFDS, 2023; SFA, 2023; USDA and FSIS, 2023). These regulations are overseen by national agencies in each country (Table 1).
| Regions/countries | Departments | Policies/regulations | References |
|---|---|---|---|
| Australasia | Food Standards Australia New Zealand (FSANZ) | Cultured quail as a novel food (food standards code, Applications No. A1269) | FSANZ, 2023 |
| European Union (EU) | European Parliament (EP) | European Parliament and of the council (No. 2015/2283) | EU, 2021 |
| Korea | Ministry of Food and Drug Safety (MFDS) | Temporary standards and recognition standards of specification for food, etc. (No. 2023-507) | MFDS, 2023 |
| Singapore | Singapore Food Agency (SFA) | Requirements for the safety assessment of novel foods and novel food ingredients (revised on July 20, 2023) | SFA, 2023 |
| United Kingdom | Food Standards Agency (FSA) | Cell-cultivated products (revised on November 16, 2023) | FSA, 2023 |
| United States | United States Department of Agriculture-Food Safety and Inspection Service (USDA-FSIS) | FSIS responsibilities in establishments producing cell-cultured meat and poultry food products (No. 7800.1) | USDA and FSIS, 2023 |
| Food and Drug Administration (FDA) | Federal Food, Drug, and Cosmetic Act (U.S. Code: Title 21) Public Health Service Act (U.S. Code: Title 42) Fair Packaging and Labeling Act (U.S. Code, Title 15) |
FDA, 2019; FDA, 2023b |
As there are many threats to the safety of cultured meat, it is essential to establish a “standard safety assessment procedure for cultured meat” that includes not only cell-cultured chicken but also other major livestock species, such as beef and pork, or cell-cultured seafood, to ensure the safety certification and commercialization of cultured meat (Ong et al., 2021). Furthermore, potential threats in cultured meat that are not yet well understood need to be further investigated, and the safe production of cultured meat should be based on the use of validated food ingredients in the product production process and appropriate regulations (Bhat et al., 2015). Safe consumption of the product is a prerequisite for cultured meat to be licensed and marketed as a new food, which requires standardization of the manufacturing process or the development of manufacturing guidelines (Mariano et al., 2023). It may also be necessary to evaluate the safety of the final product manufactured according to the standardized process or manufacturing guidelines, which can be done using methods similar to those used to evaluate new foods for authorization for human consumption (Lee et al., 2023c). In general, the safe consumption of food is assessed by short-term and long-term toxicity tests in laboratory animals.
Toxicity tests used to assess food for human consumption analyze the genotoxicity, reproductive toxicity, hematotoxicity, hepatotoxicity, or allergenicity in comparison to existing products. In assessing the safety of cultured meat for consumption, it may be necessary to standardize or set guidelines for the following five processes:
Sustainability and Animal Welfare
According to the United Nations’ World Population Prospects 2022 report (UN, 2022), the world’s population is expected to reach 9.7 billion by 2050 from 8 billion in 2022, and a joint report prepared by the Organization for Economic Co-operation and Development (OECD) and the FAO (OECD and FAO, 2022) predicts that global meat consumption will increase by 15% by 2031 to keep pace with projected population growth. As a result, more land for growing feed is needed to keep up with the trend of increasing meat consumption.
The global livestock industry has drawn increased attention in recent years because of the magnitude of its environmental impact. Greenhouse gases from livestock production are estimated to be 14.5% of global greenhouse gas emissions, and agricultural water use is reported to be 29% of global water use, 98% of which is used for the production of animal feed (Gerber et al., 2013; Mekonnen and Hoekstra, 2012). The environmental costs of livestock production also include land degradation, eutrophication of lakes and rivers, lower soil fertility, reduced biodiversity, increased exposure to zoonotic diseases, and accumulation of livestock manure, which could contaminate surface and groundwater, and has been shown to contribute to the transmission of zoonotic diseases and antibiotic-resistant bacteria (Godfray et al., 2018; Morand et al., 2019; Xie et al., 2018; Young et al., 2014).
Cultured meat has been reported to involve 78%–96% less greenhouse gas emissions, 99% less land use, 82%–96% less water, and 7%–45% less energy use than conventional meat production methods, depending on the specific meat product being compared and the species involved (Reis et al., 2020; Tuomisto and Teixeira de Mattos, 2011). These data suggest that cultured meat could be a key promotional tool to induce positive consumer perceptions of its environmental benefits and engagement in environmental protection. Pakseresht et al. (2022) reviewed a total of 43 articles and identified environmental and ethical concerns among eight major factors determining the consumer acceptance of cultured meat. However, data quantifying the climate and environmental impacts of cultured meat production is highly speculative, based on forward-looking projections, and actual cultured meat production systems are often hidden due to intense competition, leaving little detailed information available for analysis (Lynch and Pierrehumbert, 2019; Tuomisto, 2018). Therefore, a systematic approach with a larger sample size of cultured meat production technologies needs to be developed to assess the environmental impact of cultured meat.
As global meat consumption is on the rise, the scale of farming and the number of animals slaughtered are expected to increase, and the religious, ethical, and environmental controversies that arise from the slaughter process are likely to become more intense than before (Heidemann et al., 2020). Over the years, continued efforts have been made to improve the efficiency of the livestock industry for mass production, but equally important is prioritizing animal welfare and, accordingly, the movement to improve animal welfare, such as developing standards for animal welfare certification and labeling schemes, is reported to be increasing every year (Anomaly, 2015; Parker et al., 2017). Given that cultured meat would reduce the need for raising livestock for slaughter, it can improve animal welfare concerns (Hocquette, 2016), and studies of consumers suggest that the emotional benefits of cultured meat in terms of animal welfare contribute to positive perceptions of cultured meat (Bryant and Barnett, 2020; Lin-Hi et al., 2023; Rolland et al., 2020). Conversely, some consumers have expressed concerns that cultured meat will affect the demand for industrial animals, leading to a decrease in the number of live animals, which poses a potential threat to traditional livestock farming, ultimately leading to a disruption of the balance between animals and nature (Laestadius and Caldwell, 2015; Newton and Blaustein-Rejto, 2021). In response to these concerns, scenario analysis studies have been conducted on the possibility of cultured meat partially replacing traditional livestock farming, but cultured meat is still considered to be at a technological plateau, requiring extensive research and large capital investments to replace conventional meat production (Mateti et al., 2022; Moritz et al., 2023).
Consumer Perception
Cultured meat producers are emphasizing the benefits of environmental efficiency, sustainability, eco-branding, and environmental costs to win over consumers and are actually creating added value for cultured meat products by reducing the negative environmental impact of product production and providing differentiated products that make consumers feel like they are investing in environmental protection (Reis et al., 2020). In the review by Pakseresht et al. (2022) mentioned above, 43 (17.7%) of 243 screened articles on cultured meat development and technology concerned consumer attitudes, highlighting the scarcity of studies exploring consumers responses to this technology. In a choice experiment using a randomized group of 533 consumers, it was found that taste, health, price, animal welfare, environmental impact, and social impact were the most important factors in determining the purchase of a burger product and that only 11% of consumers would choose a burger made with cultured meat if all burgers had the same price (Slade, 2018). However, when presented with a positive framing of cultured meat, more than 66%–70% of consumers were willing to try or purchase cultured meat, and those who were willing to purchase had a favorable evaluation of cultured meat, citing improvements in environmental and animal welfare as benefits of cultured meat (Bryant and Barnett, 2020; Rolland et al., 2020; Wilks and Phillips, 2017; Zhang et al., 2020). Furthermore, in a system dynamics model study to estimate the demand for cultured meat, the price of the product had the greatest impact on the speed of promotion and purchase decision-making for cultured meat, with low prices showing high demand regardless of the promotion strategy, suggesting the importance of proper pricing in the launch of cultured meat products (Skinner and Blake, 2023).
However, cultured meat is categorized as a novel food that is only available for purchase or tasting in limited quantities in a handful of countries, and all the consumer research published as of November 2023 is based on hypothetical product settings. Additionally, consumer response has been found to be largely consistent, and it is expected that the production of affordable cultured meat to meet consumer satisfaction will be paramount. As alluded to above, another consumer concern regarding the development of the cultured meat industry is that it will negatively impact traditional livestock farmers (Wilks and Phillips, 2017). A survey of the acceptance of alternative meat products among farmers and non-farmers found that both farmers and non-farmers expressed concerns about the impact of cultured meat on traditional livestock farming, with farmers reporting a lower preference for alternative meat products than non-farmers (Crawshaw and Piazza, 2023).
Cultured meat also provokes ethical, cultural, and religious discussions. According to Islamic beliefs, halal means exception in Arabic, and whether cultured meat is halal is a determining factor in Muslims’ acceptance of cultured meat consumption (Hamdan et al., 2018). Muslims in the United Kingdom were less likely to try new foods than non-Muslims due to uncertainty about halal status, but Muslims were found to be more likely to purchase cultured meat than non-Muslims (Boereboom et al., 2022). Muslims in Singapore also considered the safety and halal status of cultured meat before accepting it, and there was a link between food safety and religious acceptance (Ho et al., 2023). To enter the kosher and halal markets, cultivated meat must comply with specific standards and requirements, including those related to its origin and method of production. In September 2023, Orthodox Union Kosher, the world’s largest and most influential kosher certification authority, certified poultry products from SuperMeat as kosher, marking a major advancement for the food technology’s acceptance under Jewish dietary law (Tress, 2023). At the time of writing, Aleph Farms (the first to receive approval for cultured meat for a bovine species) is awaiting a decision on kosher and halal certification of its beef steaks after seeking consultation from several religious authorities.
Therefore, the strategies necessary for consumer acceptance of cultured meat must consider the positions of various sectors, such as government policy, food safety, traditional livestock farming and cultured meat, and religious/cultural/ethical perspectives. Accurate data and research are needed to compare the sustainability of the conventional meat industry and cultured meat industry, not only to highlight the positive aspects of cultured meat but also to consider the coexistence of traditional livestock farming and cultured meat (Bryant and Barnett, 2018). However, market-based information on actual cultured meat technologies is inconsistent, making it difficult to evaluate and analyze, and environmental impact analysis is based on data with higher uncertainty compared to traditional livestock farming (Rodríguez Escobar et al., 2021). In addition, because most consumers’ positive perception and acceptance of cultured meat is based on trust in the government, it is necessary to establish strict standards for food safety (Ho et al., 2023).
In conclusion, to assess the ideal sustainability of cultured meat, bridging the knowledge and information gap is a must, and collaboration between relevant companies and researchers is needed to integrate the entire production process and scenarios so that the environmental impact of cultured meat can be reasonably predicted. Furthermore, the government should take into account the proposed scenarios and establish regulations to enable consumers to choose safe cultured meat.
Domestic and International Cultured Meat Companies
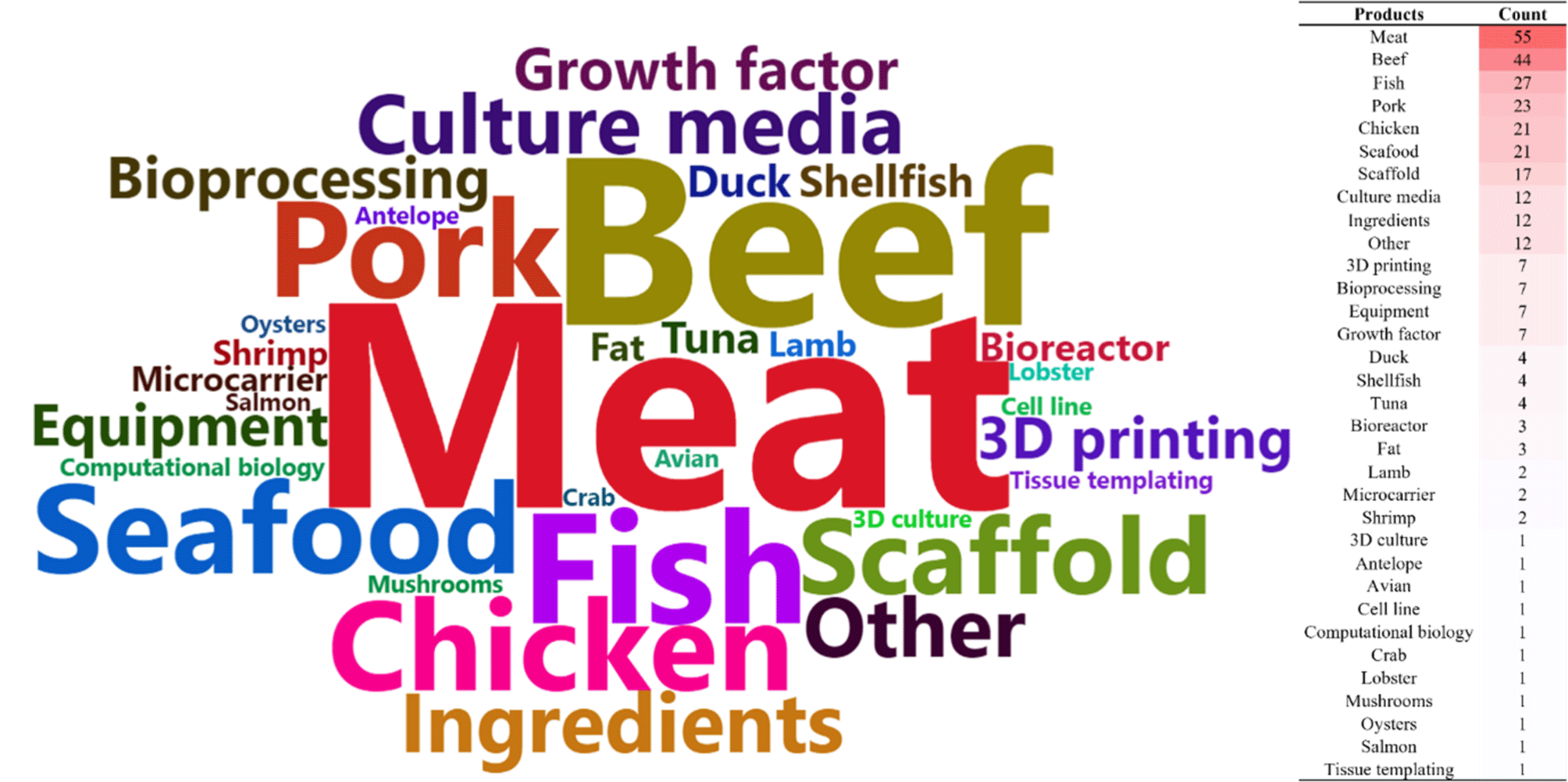
Information on domestic and international cultured meat companies as of 2023 is presented in Table 2, with a total of 195 companies producing food-grade cultured meat-related products in 35 countries. The largest number of cultured meat companies were identified in the United States (53), followed by the United Kingdom (17), Israel (14), Singapore and Canada (11), South Korea (10), Germany (9), the Netherlands and Japan (6), India, France and mainland China (5), South Africa, Argentina, and Australia (4), the Czech Republic (3), Belgium, Switzerland, Spain, Austria, and Chile (2), and other countries (New Zealand, Denmark, Russia, Malaysia, Mexico, Vietnam, Sweden, Iceland, Croatia, Turkey, and Portugal). Furthermore, of the 307 product categories mentioned as being researched by companies, the top 10 categories are meat, beef, fish, pork, chicken, seafood, scaffold, culture media, ingredients, and others, accounting for 79.5% of the total, indicating that current trends in company-level cultured meat research are centered on cultured meat (beef>fish>pork>chicken=seafood), supports, and media (Fig. 1). However, it is necessary to be cautious in identifying trends as there are many cases where the researchers do not clearly mention the animal species under research and refer to it as meat or seafood.
Production of Cultured Meat
MSCs are muscle-derived adult stem cells that are responsible for the regenerative capacity of muscle following damage to myofibers. MSCs are characterized by rapid proliferation in a highly active state early in life, while the proportion entering a quiescent state increases with age (Mesires and Doumit, 2002). Myofibrils are composed of structures surrounded by an inner sarcolemma and an outer basement membrane, and the basal lamina, which is close to the myofibrils, has been identified as an extracellular matrix (ECM) that is in direct contact with MSCs and is involved in the maintenance of physiological functions and the development of skeletal muscle (Holmberg and Durbeej, 2013; Zhang et al., 2021). The basal lamina is composed mainly of type IV collagen, which plays a role in maintaining MSCs in a quiescent state by sequestering various growth factors and signaling molecules involved in their activation and proliferation (Kann et al., 2021; Sanes, 2003). Furthermore, quiescent MSCs located in the niche between the basal lamina and myofibrils have a fusiform morphology with little cytoplasm and organelles and have been shown to express MSC-specific genes, such as paired box protein 3 (Pax3) and Pax7, and myoblast determination protein 1 (MyoD) at the beginning of quiescence or proliferation entry (Fu et al., 2015; Kuang et al., 2006; Zhang et al., 2010).
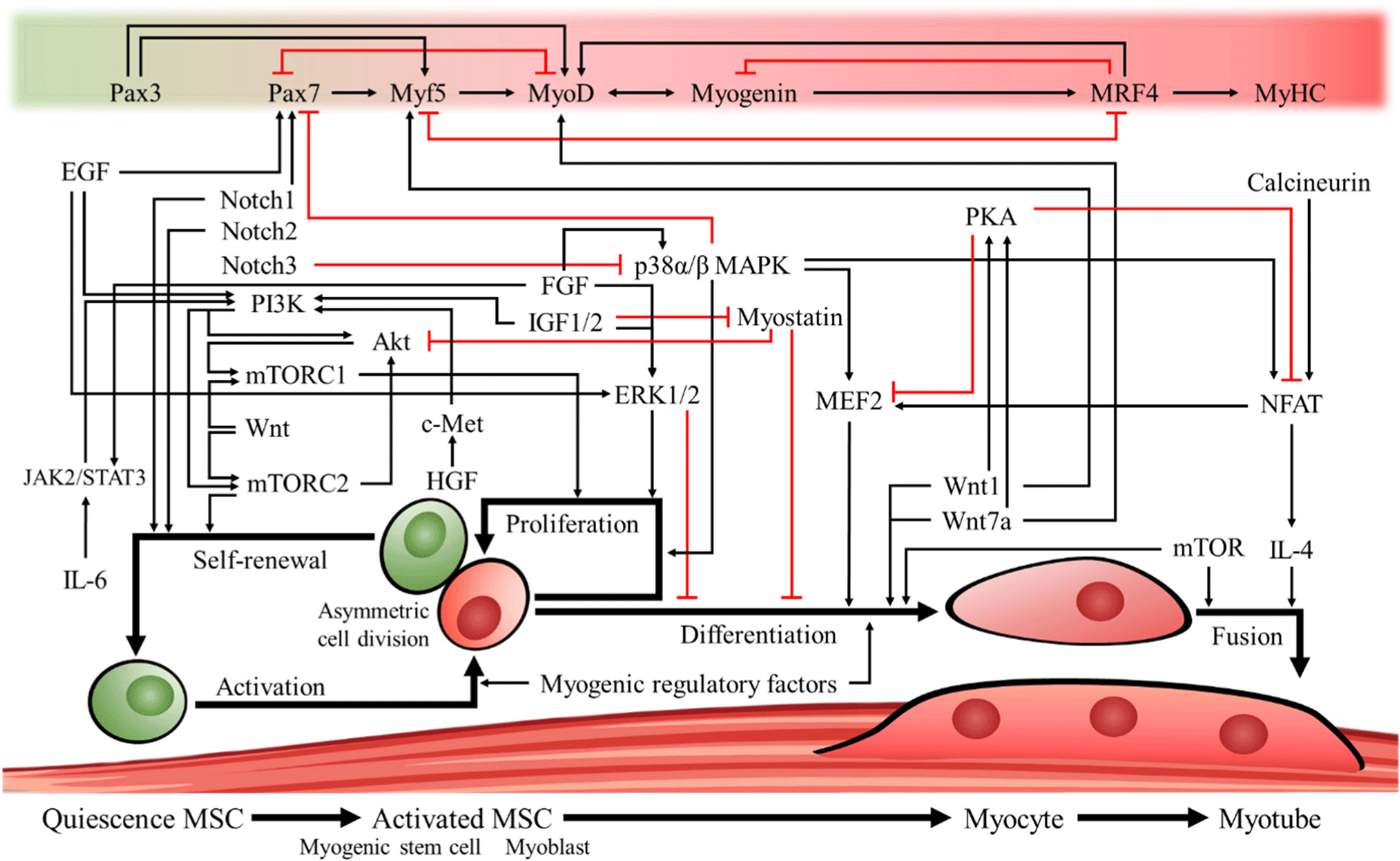
Understanding the regeneration process of MSCs is necessary for cultured meat production, and the genes and signal transduction pathways that regulate proliferation and differentiation that have been widely reported to date are shown in Fig. 2. Pax3 is considered one of the important genes responsible for MSC survival during embryogenesis. It is also purported to be involved in the formation and underlying development of early muscles by affecting the expression of MyoD and myogenic factor 5 (Myf5) to regulate the development of limb muscles (MyoD) and peri-spinal and intercostal muscles (Myf5) in early embryos (Kablar et al., 1997). Pax7 is an essential gene for MSC maintenance, and individuals with Pax7 knockout show a decreased rate of muscle regeneration in muscle injury treatments and difficulty in generating MSCs (Kuang et al., 2006). In addition, Pax7 has been found to act as an antagonist of MyoD, resulting in an increased number of Pax7-positive cells in the muscles of individuals with MyoD knockout (Kuang et al., 2006; Olguin and Olwin, 2004; Seale et al., 2000).
Activation of MSCs is an early step in myogenesis. When a muscle is damaged, the disruption of the basal plate and reorganization of the environment leads to interactions between signaling molecules that were previously sequestered by the basal plate and MSCs, leading to their activation (Li et al., 2018). Muscle formation is mainly regulated by myogenic regulatory factors (MRFs) expressed in activated MSCs. Some representative MRFs are MyoD, Myf5, myogenin, and muscle-specific regulatory factor 4 (MRF4, also known as Myf6; Kim et al., 2023a). Activated MSCs divide to produce satellite cell-derived myoblasts that continue to divide and proliferate before committing to differentiation and fusing to form myotubes, which then mature into myofibers. When satellite cells are activated, they initiate differential expression of MRFs depending on the asymmetry of cell orientation after division (Kuang et al., 2007). Accordingly, it has been shown that if the orientation of the cells formed after somatic cell division is on the myofibrillar side, they upregulate origin regulatory factors, such as MyoD and Myf5, whereas cells on the basal plate side do not express Myf5 and retain stemness (Kuang et al., 2007; Troy et al., 2012).
MyoD and Myf5 are genes that activate myogenin and MRF4 and participate in the late stages of muscle formation by influencing the fusion of myoblasts and the initiation of their final differentiation, leading to cell maturation and ultimately the formation of multinucleated myotubes (Cornelison et al., 2000; Hawke and Garry, 2001; Punch et al., 2009; Smith et al., 1993). MyoD has somewhat overlapping roles with myogenin, but when myogenin is deleted, MyoD is unable to take over its role, and individuals with myogenin deletion have been shown to die at birth due to impaired skeletal muscle formation (Adhikari et al., 2021; Nabeshima et al., 1993). It was also found that in C2C12 cultures with myogenin deletion, myomaker and myomixer, two genes that regulate the fusion of skeletal muscle, were significantly downregulated, leading to the inhibition of differentiation (Adhikari et al., 2021). MRF4 is an origin regulator that is predominantly expressed in fully differentiated muscle fibers and plays a role in maintaining the MSC pool. It has been reported that deletion of MRF4 can significantly reduce the number of Pax7-positive MSCs in postnatal individuals (Lazure et al., 2020).
Signals that regulate the stemness of MSCs are known to include p38α/β mitogen-activated protein kinase (MAPK) or Notch. First, inhibition of p38 has been reported to induce self-renewal of MSCs by blocking the MyoD expression pathway and maintaining Pax7 expression along with inhibition of cell cycle entry to sustain an undifferentiated and proliferative state (Ding et al., 2018; Li et al., 2023; Troy et al., 2012). Among Notch signaling components, Notch1 is activated upon muscle injury in vivo by binding to myofilament ligands to induce cell cycle exit, Notch2 is activated in MSCs to maintain the stemness of the MSC population by inhibiting differentiation, and Notch3 has been shown to inhibit the p38α/β MAPK pathway to suppress myocyte enhancer factor 2 (MEF2) expression associated with differentiation (Conboy and Rando, 2002; Gagan et al., 2012; Jo et al., 2022).
It has been reported that activated MSCs are proliferation-induced and differentiation-inhibited by the phosphoinositide 3-kinase (PI3K)/Akt pathway or the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway (Li et al., 2023; Mohammadabadi et al., 2021; Ohashi et al., 2015). Growth factors known to be involved in PI3K/Akt activation include fibroblast growth factor (FGF), insulin-like growth factor (IGF)-1/2, hepatocyte growth factor (HGF)/c-Met, epidermal growth factor (EGF), and interleukin-6/Janus kinase 2/signal transducer and activator of transcription 3 (IL-6/JAK2/STAT3). These factors have been shown to act as activators of mammalian target of rapamycin complex 1 (mTORC1), which can regulate the proliferation of muscle progenitor cells (Brandt et al., 2018; Holterman and Rudnicki, 2005; Lu et al., 2017; Messersmith et al., 2021; Ohashi et al., 2015; Ornitz and Itoh, 2015; Relaix et al., 2021; Rhoads et al., 2016; Wang et al., 2023a). Furthermore, it has been confirmed that EGF and FGF are involved in the ERK1/2 pathway, one of the MAPK family signaling pathways, which can activate myoblast proliferation and impair the initiation and maintenance of differentiation (Li et al., 2023; Mohammadabadi et al., 2021; Ohashi et al., 2015). Additionally, the Wnt pathway can activate both mTORC1/2, with mTORC1 regulating metabolism in response to environmental factors (growth factors, amino acids, energy, and stress) and mTORC2 involved in the maintenance of MSC populations through phosphatase family pathways (Oh and Jacinto, 2011; Rion et al., 2019; Wei et al., 2019).
The p38α/β MAPK pathway activates MEF2 and plays a major role in the differentiation of myoblasts. Myotube formation is inhibited when MEF2 is removed because of the involvement of MEF2 in the proliferation and differentiation of MSCs (Chen et al., 2017; Shao et al., 2022; Wang et al., 2018). Furthermore, when the mTOR pathway is inhibited by rapamycin in MSC cultures, the expression of myogenic genes (Pax7, Myf5, and MyoG) is inhibited, indicating that the mTOR pathway is essential for the proliferation and differentiation of MSCs (Zhang et al., 2015). In addition, previous studies on MSC differentiation have shown that Wnt1 and Wnt7a signaling, along with activation of the Wnt/β-catenin pathway, increases β-catenin to induce myogenic differentiation of mesenchymal stem cells and activate the myogenic regulators Myf5 and MyoD to influence skeletal muscle development (Eng et al., 2013; Zhu et al., 2022). Signals that inhibit differentiation include ERK, myostatin, and protein kinase A (PKA), with myostatin reported to inhibit muscle formation by co-inhibiting the Akt pathway and PKA reported to induce proteolytic cleavage to produce factors that inhibit MEF2 signaling (Backs et al., 2011; Mohammadabadi et al., 2021; Trendelenburg et al., 2009).
The nuclear factor of activated T-cells (NFAT) can activate signaling molecules that regulate the fusion of myoblasts and myotubes, such as MEF2 and IL-4, by the calcineurin and p38/MAPK pathways; however, PKA has been reported to prevent premature differentiation of myoblasts by rephosphorylating MEF2 and NFAT while inhibiting their differentiation (Horsley et al., 2003; Knight and Kothary, 2011; McKinsey et al., 2002; Stork and Schmitt, 2002; Wu et al., 2007; Yue et al., 2023). In addition, it has been shown that mTOR regulates the proliferation of MSCs but can also regulate myotube fusion by both kinase-dependent and -independent pathways (Park and Chen, 2005).
In conclusion, an understanding of the various gene expression and signaling processes within MSCs for cultured meat production is required, and further research is needed to control and regulate cell cycle arrest and activation, proliferation, differentiation, and even fusion.
MSCs can be obtained by biopsy of muscle tissue from living animals and by harvesting muscle tissue from animals immediately after slaughter. The most used processes for harvested muscle tissue are disinfection, removal of fat and connective tissue, fragmentation, digestive enzyme treatment, sequential filtration, centrifugation, pre-culture, and finally, cell recovery to obtain primary cells (Lee et al., 2021). The obtained primary cells are then subjected to immunofluorescence staining or polymerase chain reaction to determine the proportion of MSCs from the proportion of progenitor regulatory factors in the primary cells. Typically, Pax7 and MyoD are used to determine the purity of MSCs, and by comparing their expression levels, the activation of the MSCs used in the experiment can be determined (Ding et al., 2017; Kim et al., 2023a; Pasut et al., 2013). In addition, flow cytometry methods, such as fluorescence-activated cell sorting (FACS) and magnetic-activated cell sorting (MACS), can be used to obtain pure MSCs labeled with MSC-specific markers, which can then be proliferated to sufficient quantities for use in cultured meat experiments and production (Ding et al., 2018; Gromova et al., 2015; Kim et al., 2023a; Motohashi et al., 2014).
The culture of MSCs has been performed since before the 1990s, and the methods can be broadly divided into two types: culture of isolated single strands of muscle fibers and culture of cells isolated from enzymatically treated muscle tissue (Anderson and Pilipowicz, 2002; Bischoff, 1986; Doumit and Merkel, 1992; McFarland et al., 1988). Fetal bovine serum (FBS) is a key ingredient added to the basal medium for culture, but the exact nature of FBS is still poorly understood, and commercialization of cultured meat is currently limited by the need to replace FBS completely (Lee et al., 2022; Lee et al., 2023a). It is difficult to avoid the ethical issues associated with the production of FBS, as more than 2 million bovine fetuses derived from slaughtered mothers are used for FBS production each year (Lee et al., 2022). In addition to ethical concerns, the high price of FBS has led numerous research teams to investigate serum-free media as an alternative to FBS, and along with research to refine the active ingredients of FBS, results support that serum can be effectively replaced with proteins required for cell growth or a combination of various growth factors (Messmer et al., 2022; Schenzle et al., 2022; Skrivergaard et al., 2023; Stout et al., 2022; Stout et al., 2023). Furthermore, to meet halal standards, the use of blood in cultured meat production is also limited (Hamdan et al., 2018). However, challenges remain, such as the use of recombinant growth factors in the preparation of serum-free media or chemically composed media and the cost of expensive additives (Stout et al., 2022; Stout et al., 2023).
Once the medium in which the cells are to be cultured is prepared, the method of culturing the cells must be chosen according to each cell type. Cell culture techniques for cultured meat production can be broadly divided into adherent culture and floating culture, and among the cells, MSCs and fibroblasts have been studied, as well as adipocytes (Bodiou et al., 2020; Ge et al., 2023; Humbird, 2021; Lee et al., 2021). Approximately 1014 cells and 10,000 L of culture medium are required to produce 1 t of cultured meat, assuming a cell density of 107 cells/mL in the bioreactor (Guan et al., 2021). However, the larger the bioreactor size, the higher the stirring intensity needed to maintain a homogeneous environment in the vessel, which can lead to shear stresses of a magnitude that can cause cell damage (Allan et al., 2019). In a modeling study of cultured meat production scenarios, it was emphasized that optimal cell selection to reduce the consumption rate of medium, completely replace or decrease the cost of growth factors, and increase the size of perfusable bioreactors are necessary for mass production environments (Risner et al., 2021).
As such, cultured meat is a tissue engineering technique under investigation based on the theory that the self-renewal ability of MSCs can be harnessed to produce dozens of times the amount of muscle tissue from a small piece of muscle. Cultured meat is one of the promising future technologies that can be used as an important source of meat for some countries because it is less sensitive to climatic conditions than conventional meat production, but there is a need to improve economic issues, such as cell acquisition, mass production and cost, and the amount of culture fluid and energy required for production compared to real meat. Additionally, research is being conducted worldwide to improve the qualitative limitations, such as flavor, texture and structure, meat color, and nutritional content, which are different from those of real meat.
Recent Trends in Muscle Satellite Cell Culture Technologies
Bovine MSC isolation techniques for cultured meat production reported in 2023 are shown in Table 3. The goal of the isolation process is to obtain the raw material for cultured meat. The isolation techniques used can be broadly categorized into 1) enzymatic reactions and centrifugation to obtain MSCs and pre-culture and 2) flow cytometry to increase the purity of the MSCs.
| Muscles | Enzymes | Centrifugation conditions | Pre-plating | References |
|---|---|---|---|---|
| Longissimus | Pronase | 500×g, 10 min | N/A | Kim and Kim, 2023 |
| Biceps femoris | Pronase | 300×g, 5 min; 1,200×g, 15 min | N/A | Kim et al., 2023a |
| Longissimus | Pronase | 500×g, 10 min | N/A | Kim et al., 2023b |
| Longissimus thoracis | Collagenase II, Dispase II | N/A | 3 h+3 h | Lee et al., 2023b |
| Semitendinosus | Collagenase | N/A | N/A | Messmer et al., 2023 |
| Top round | Collagenase mix | 800×g, 5 min | N/A | Park et al., 2023 |
| Semimembranosus | Collagenase, trypsin | 100×g, 5 s; 1,000×g, 10 min | N/A | Skrivergaard et al., 2023 |
| Semitendinosus | Collagenase II | N/A | N/A | Stout et al., 2023 |
| Longissimus thoracis | Collagenase, trypsin | N/A | 1 h | Tzimorotas et al., 2023 |
| Longissimus lumbrorum | Collagenase D | 76×g, 5 min | 1 h | Uyen et al., 2023 |
| Hind limb | Collagenase II, trypsin | N/A | N/A | Zhang et al., 2023 |
| Longissimus dorsi | Collagenase II | 500×g, 10 min | 1 h | Zygmunt et al., 2023 |
First, an enzymatic reaction is performed to obtain primary cells from muscle tissue. The cells are minced to increase the surface area, and connective tissue is removed to facilitate the reaction. Enzymes used for MSC isolation include collagenase, dispase, trypsin, and pronase in various concentrations. Centrifugation is a method that uses centrifugal force and density gradients to remove unwanted tissue and isolate desired cells. In the isolation process of MSCs, the centrifugal acceleration was 76–1,200×g, and the time was generally around 5–15 min.
The cells obtained by enzymatic reaction and centrifugation are primary cells. Cell pre-plating or purification techniques, such as FACS and MACS, are employed to increase the purity of MSCs. Pre-culture is a technique for isolating specific cells from a mixture of different cell types, effectively increasing the purity of MSCs by exploiting differences in the adhesion properties of primary intracellular fibroblasts and MSCs (Richler and Yaffe, 1970). The preincubation time used in the isolation of bovine MSCs reported in 2023 was 1–3 h. Fibroblasts begin to adhere 5 min after incubation and adhere to surfaces faster than MSCs, indicating that a relatively high purity of Pax7- or MyoD-positive cells can be obtained using the preincubation process (Table 3; Kim et al., 2022; Xu et al., 2018; Yoshioka et al., 2020). In other studies, preincubation conditions have been shown to vary from 5 min to 24 h after fibroblasts begin to adhere (Table 3). One of the effective methods for rat MSCs was preincubation for up to 10 min with shaking every 5 min (Yoshioka et al., 2020). For chicken MSCs, it was up to 2 h with shaking every 8 min after 2 h of rest, indicating that the preincubation conditions may also vary depending on species-specific cell characteristics (Kim et al., 2022).
Even without the pre-culture step, the purity of the MSCs can be increased by using cell sorting techniques, such as FACS and MACS. Some drawbacks of the flow cytometry-based isolation process are that it requires expensive equipment and reagents, trained professionals, and is cumbersome because of sorter-induced cellular stress, such as high-pressure jets, high voltage, and laser exposure during the isolation process, and cytotoxicity that can occur when using specific markers (Lopez and Hulspas, 2020). Although FACS has the advantage of being able to separate cells based on their size or three-dimensional features using fluorescent labeling, it has the disadvantage of expensive equipment and long analysis times. MACS uses magnetic particles to sort cells more than four times faster than FACS and is less expensive, but it is difficult to apply to cells that are susceptible to magnetism or cannot be labeled (Gerashchenko, 2011).
Both FACS and MACS label cells with clusters of differentiation (CD), which are specific markers of MSCs, and each uses a fluorescent agent for FACS and magnetic particles for MACS. Specific markers for MSCs used for cell labeling are species-specific, but some examples are integrin α7, vascular cell adhesion protein 1 (Vcam1), and differentiation clusters, such as CD29 (integrin β1), CD34 (hematopoietic stem cell marker), CD56 (neural cell adhesion molecule), and CD82 (4-transmembrane glycoprotein) (Castiglioni et al., 2014; Uezumi et al., 2016; Yoshioka et al., 2020). After sorting the MSCs, they can then be cultured to check the expression of Pax7 or MyoD to confirm the purity of the isolated MSCs, and the proliferation and differentiation capacity of the cells can be assessed.
In conclusion, the cell biological characteristics necessary for the isolation of MSCs from each animal species have not yet been fully identified, and comprehensive research is limited by the lack of standardization of separation methods, which is an obstacle to industrialization.
MSCs obtained during the isolation process will multiply in number in a properly conditioned growth medium. The proliferation process is directly related to the yield of cultured meat, and various studies have been conducted to improve the proliferation efficiency. First, the basal media commonly used for MSC culture are Ham’s F-10, Dulbecco’s modified Eagle’s medium (DMEM), and DMEM/F12, with bovine fetal serum added to the media at a concentration of 10%–20% (v/v) in most cases (Table 4). Basal media is a solution of basic nutritional components (e.g., amino acids, glucose, lipids, nucleic acid bases, inorganic salts, vitamins, buffers, pH indicators) formulated in a certain proportion according to the culture conditions of the desired cells. In the culture of MSCs, the basal medium and serum concentrations are known to be closely related to the cell proliferation rate and myotube formation (McFarland et al., 1988). In a broiler MSC culture experiment based on culture medium composition, DMEM was found to be more effective than McCoy’s 5A medium in terms of proliferation rate and MRF expression (Flees et al., 2022). In addition, the common view that a low glucose content is effective for chicken and bovine MSC proliferation when using DMEM as basal medium was confirmed (Flees et al., 2022; Zygmunt et al., 2023).
| Basal media | Sera | Growth factors | Coatings | References |
|---|---|---|---|---|
| DMEM | 10% FBS | N/A | N/A | Kim and Kim, 2023 |
| DMEM/F12 | 10% FBS | N/A | N/A | Kim et al., 2023a |
| DMEM | 10% FBS | N/A | N/A | Kim et al., 2023b |
| Ham's F-10 | 20% FBS | bFGF | Collagen I | Kim et al., 2023c |
| Ham's F-10 | 20% FBS | bFGF | Collagen, Matrigel | Oh et al., 2023 |
| Ham's F-10 | 20% FBS | N/A | Bovine collagen I, Matrigel | Park et al., 2023 |
| DMEM/F12, Ham’s F-10 | Serum-free media, 20% FBS | bFGF, HGF, Hydrocortisone, IGF-1, IL-6, ITSE, PDGF, VEGF | Fibronectin, Laminin | Messmer et al., 2023 |
| DMEM, DMEM/F12 | 10% FBS, Serum-free media | bFGF, Fetuin, ITS, HGF, PDGF, Insulin | Matrigel | Skrivergaard et al., 2023 |
| DMEM | 20% FBS | bFGF | Laminin, Vitronectin | Stout et al., 2023 |
| LG-DMEM | 10% FBS, 2% FBS, Ultroser G | N/A | Entactin-Collagen-Laminin | Tzimorotas et al., 2023 |
| DMEM | 15% FBS | N/A | Rat tail collagen I | Uyen et al., 2023 |
| DMEM | 20% FBS | bFGF | N/A | Zhang et al., 2023 |
| LG-DMEM, HG-DMEM | 20% FBS | bFGF | Gelatin | Zygmunt et al., 2023 |
DMEM, Dulbecco’s modified Eagle’s medium; DMEM/F12, Dulbecco’s modified Eagle’s medium and Ham’s F-12 Nutrient Mixture; LG-DMEM, low glucose-DMEM; HG-DMEM, high glucose-DMEM; FBS, fetal bovine serum; bFGF, basic fibroblast growth factor; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; IL-6, interleukin-6; ITS, insulin-transferrin-selenium; ITSE, insulin-transferrin-selenium-ethanolamine; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; N/A, not applicable.
As adherent cells, MSCs require an ECM-based coating for proliferation and differentiation. Representative ECMs used for bovine MSC proliferation have been shown to be gelatin, collagen I, laminin, and Matrigel (Table 4). Integrin α7β1, which is present in the cell membrane of MSCs, binds to collagen and laminin, and laminin induces the proliferation and migration of satellite cells (Öcalan et al., 1988; Sanes, 2003). However, C2C12 cells cultured on plates coated with ECM proteins had a better proliferation rate compared to highly elastic coatings, such as collagen I/laminin/fibronectin hydrogels, which were not conducive to inducing proliferation of MSCs (Palade et al., 2019).
Growth factors are cell signaling proteins. For MSCs, basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and HGF are commonly used in culture (Table 4). Typically, bFGF is added to the proliferation medium at a concentration of 5–10 ng/mL. Accordingly, in bovine MSC cultures, a bFGF content of 10 ng/mL in the medium led to a faster proliferation rate than when the bFGF content was 5 ng/mL (Zygmunt et al., 2023). In addition, the expression of various endothelial cell-derived growth factors (IGF-1, HGF, bFGF, and VEGF) can stimulate MSCs to proliferate and regenerate muscle (Yamamoto et al., 2020; Zygmunt et al., 2023).
However, research into cultured meat using FBS remains prevalent. Even when serum-free media is used, various culture ingredients, such as basal media, coating agents, and recombinant proteins (growth factors, hormones), are employed. To address this issue, the development of natural product-derived media that meet food regulatory requirements is underway. However, industrialization is inevitably delayed because companies and research teams cannot disclose it due to competitiveness.
MSC differentiation is commonly achieved by removing the proliferation medium and replacing it with the differentiation medium once the cells have reached a sufficient cell density in the proliferation medium (Ding et al., 2017). The differentiation process typically uses media containing 2% FBS or horse serum to induce serum starvation (Table 5). Serum starvation is often chosen to induce differentiation of MSCs (Pirkmajer and Chibalin, 2011). Induction of differentiation in studies published in 2023 was mainly performed at cell densities above 70% confluence, and the duration of differentiation varied by 1–10 d depending on the experimental conditions, but only one study was identified that varied the serum concentration within the culture period (Table 5).
| Basal media | Sera | Time (d) | References |
|---|---|---|---|
| SILAC DMEM Flex Media | 2% HS | 4 | Kim and Kim, 2023 |
| DMEM/F12 | 2% HS | 1–4 | Kim et al., 2023a |
| DMEM | 2% FBS | 1–4 | Kim et al., 2023c |
| DMEM | 2% HS | 6 | Lee et al., 2023b |
| DMEM/F12 | Serum-free | 3 | Messmer et al., 2023 |
| DMEM | 2% FBS | 4–5 | Oh et al., 2023 |
| DMEM | 2% FBS | 1–6 | Park et al., 2023 |
| Neurobasal | N/A | 2 | Stout et al., 2023 |
| DMEM | 2% HS | 3 | Uyen et al., 2023 |
| DMEM | 2% HS, 10% HS | 3–7 | Yun et al., 2023 |
| DMEM | 2% HS | 1–5 | Zhang et al., 2023 |
| LG-DMEM, HG-DMEM | 20% HS | 3–10 | Zygmunt et al., 2023 |
When a low-serum environment is used to induce differentiation of MSCs, extensive changes occur at the transcript level, with upregulation of progenitor transcription factors and markers associated with differentiation identified during the differentiation process (Dmitriev et al., 2013; Messmer et al., 2022). Transient and mild levels of serum starvation (15% serum, v/v) induce autophagy, which can promote cell metabolism and differentiation, but 5% serum starvation induces excessive autophagy, leading to cell death (Wang et al., 2023b).
A hypoxic environment (1%–10% O2) in MSC culture can create conditions that mimic oxygen saturation in mature skeletal muscle. Moreover, a hypoxic environment (2% O2) upregulates the myogenic regulators Pax7, Myf5, and MyoD, and intermittent hypoxic exposure increases the expression of VEGF released from MSCs (Koning et al., 2011; Nagahisa and Miyata, 2018; Urbani et al., 2012). The hypoxia-induced factor-1 signaling pathway, which is expressed in response to hypoxic conditions, is thought to be involved in the regulation of myoblast proliferation and differentiation. Under a hypoxic environment (1% O2), broiler MSC cultures exhibited a decrease in the level of MyoD-positive cells along with changes in the transcriptome profile (Jung et al., 2024; Li et al., 2007).
However, serum starvation tends to be the preferred method for induction of differentiation compared to hypoxic environments, and the signaling pathways involved and their effects on differentiation remain poorly understood. Furthermore, the regulations regarding cultured meat are extensive and do not clearly differentiate between cultured meat with or without differentiated tissue, leading to confusion within the industry.
Conclusion
This study analyzed the current technology, industrialization level, and future prospects of cultured meat by analyzing research reports and media reports related to the industrialization of cultured meat. At present, major companies are not entering mass production except for prototype development, and the reason they do not disclose related technologies is that they do not have enough technological capabilities. Therefore, when investing in cultured meat development companies, it is necessary to accurately assess the level of technology that the company has or has acquired. Much of the focus is currently on cell acquisition technology, cell line acquisition technology, and cell culture and muscle differentiation technology. While the level of technology related to the industrialization of cultured meat has reached the stage where prototypes can be produced, it is believed that it has not yet reached the stage where production costs can be dramatically reduced and the product sold to the market. Nevertheless, given the steady increase in the number and depth of studies related to the industrialization of cultured meat and the increasing number of companies involved, it is expected that the industrialization of cultured meat could begin in the not-too-distant future.