Introduction
In vitro culture and differentiation of muscle stem cells, accompanied by flavor and texture processing, produce protein products derived from cultured muscle tissue, commonly called cultured meat (O’Neill et al., 2021). Cultivated meat has emerged as a cutting-edge approach for delivering efficient, secure, and environmentally friendly animal protein sources (Park et al., 2022). Nevertheless, the limited in vivo proliferation capacity of Yanbian bovine skeletal muscle satellite cells (BSCs), pivotal for muscle fiber production, poses a challenge hindering the industrialization of cultured meat. Furthermore, as the global population expands, traditional livestock farming may fall short of satisfying the rising demand for meat and protein-rich diets. Nowadays, artificial meat, as well as continuous innovations in the field of cellular agriculture, can better provide people with real meat products without environmental, ethical, and technological problems. Since muscle formation is completed during embryonic development in most meat animals, in vitro methods, such as myogenic cell culture and genomic techniques, can be used as a way to understand the acute regulation of myogenesis (Liu et al., 2010). To boost the proliferation of muscle stem cells in vitro, several investigations have supplemented the culture medium with up to 20% serum, along with growth factors and inflammatory mediators in certain instances (Gomez Romero and Boyle, 2023). This practice significantly adds to the current high cost of producing cell-cultured meat. However, because the majority of the small molecule compounds identified in earlier investigations were created through chemical processes, their dietary safety is unclear (Hadi and Brightwell, 2021). Hence, the present study explored the regulatory effects of L-ascorbic acid (AA), commonly known as vitamin C, a widely available water-soluble vitamin naturally present in fruits and vegetables, on muscle development when incorporated into the culture medium.
Water-soluble vitamins, particularly those belonging to the B and C groups, are deemed crucial constituents of fundamental animal cell culture media, and their significance in this context has been extensively documented (Zhitkovich, 2020). Crucially, vitamin C is indispensable for collagen synthesis by fibroblasts, a process that might be vital for maintaining the structural soundness of cell-cultured meat (Noriega-González et al., 2022). AA exhibits diverse biological functions, ranging from stimulating cell growth and differentiation to serving as a potent reducing agent and antioxidant. As it plays a crucial role in regulating numerous cellular processes across various cell types, it is essential to supplement it in the culture medium. Moreover, AA has been extensively employed in the in vitro cultivation of cells derived from pigs and chickens, specifically in facilitating the differentiation of porcine skeletal muscle satellite cells and chicken adult muscle cells (Lu et al., 2022; Zhang et al., 2019). Nonetheless, its application and research in cattle cell cultures is relatively limited.
Yanbian cattle have high meat production performance, delicate and tender meat, even and beautiful marbling, and good fat deposition effect, meat quality is better than other cattle breeds, which is an excellent choice for high-grade beef cattle (Xu et al., 2020). However, the problems of high production cost and long production cycle of high-grade beef still need to be solved. With the pursuit of high-quality diets, the choice of meat has changed from pork to beef and lamb, so research on improving the quality of beef has been an important research direction in recent years and beyond. AA has a variety of actions, including redox reactions and collagen synthesis. In addition, there is growing evidence that AA levels in the blood and other tissues are associated with growth, meat quality, and reproduction in beef cattle (Müller et al., 2018). Research conducted by Pogge and Hansen (2013) revealed that supplementing rumen-protected vitamin C to diets rich in sulfur enhanced the marbling scores in beef cattle. Fang et al. (2022) showed that AA activates the PI3K/Akt/mTOR signalling pathway via IGF1 signalling, thereby promoting the proliferation of porcine skeletal muscle stem cells. However, whether AA affects the pro-proliferative and differentiation activities of BSCs, which in turn affects meat quality as well as the mechanisms of pro-value-adding and differentiation in Yanbian cattle, is not clear.
Consequently, we initiated this study to establish an in vitro cultivation system for Yanbian cattle’s skeletal muscle satellite cells. AA was added to the culture medium under in vitro cell culture conditions, thereby investigating whether AA has a medium-optimizing effect on it. The present research sought to examine the impact of AA on the enhancement of value, differentiation, and mTOR signaling cascade in Yanbian cattle’s skeletal muscle satellite cells. We postulated that the inclusion of AA in the culture medium significantly contributes to the proliferation and differentiation of BSCs, thereby promoting protein synthesis through the Akt/mTOR/P70S6K signaling cascade. Laying the theoretical foundations and exploratory practices for large-scale cell culture muscle and preparing for the future era of cellular agriculture.
Materials and Methods
L-Ascorbic Acid 2-Phosphate Sesquimagnesium Salt Hydrate, Glycine, and serum [pregnant horse serum (HS) and fetal bovine serum (FBS)] were purchased from Sigma-Aldrich (St. Louis, MO, USA), Solarbio (Beijing, China), Gibco (San Diego, CA, USA), respectively.
Paired box 7 (Pax7), MyoG, and MyoD proteins primary antibodies and fluorescent secondary antibody (goat anti-rabbit) were purchased from BIOSS (Beijing, China). The primary antibody for MyHC protein and p-mTOR protein primary antibody was purchased from Proteintech (Chicago, IL, USA). The DAPI-containing blocking agent was purchased from Solarbio. The endoglin primary antibody was purchased from Biyuntian Company (Taiwan, China). Rapamycin was purchased from MCE (Monmouth Junction, NJ, USA). Akt, p-Akt, P70S6K, and p-P70S6K proteins primary antibodies were purchased from BIOSS.
Following the established laboratory protocol (Li et al., 2019), a 600 g muscle sample was obtained from the semimembranosus muscle of 8-day-old Yanbian calves, and connective tissue was removed. Briefly, they were rinsed three times with sterile phosphate buffered saline (PBS, Solarbio) containing 3% penicillin-streptomycin mixture (PS, Solarbio), and then they were ground into small pieces with a meat grinder and digested with 0.8% pronase (10165921001, Roche, Basel, Switzerland) for 1 h with shaking every 10 min. Centrifuged at 1,000×g for 4 min to precipitate cell clusters. Cell clusters were resuspended in a PBS wash solution containing 1% PS. Cells were collected after centrifugation at 1,000×g for 10 min and resuspended in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Thermo Fisher Scientific, Waltham, MA, USA) isolation medium containing 10% FBS (Vivacell, Shanghai, China) and 3% PS. After that, the cell suspension with 100 μm and 70 μm sieves, the cells were collected in DMEM growth medium containing 10% FBS and 1% PS after centrifuged at 700×g for 10 min. To purify the BSCs, we performed the first affixation using differential affixation centrifugation, noted as P1. After 0.5 h, the supernatant of the petri dish was aspirated and placed into a new petri dish, mixed well, and the 2nd apposition was carried out, which was recorded as P2. After 24 h, the supernatant of P2 was centrifuged at 1,000 rpm for 5 min, the supernatant was discarded, and the pelleted cells were resuspended with growth medium and transferred into a new culture dish for the 3rd affixation, which was recorded as P3 and P3 cells were replaced with new growth medium every 2 d to continue the culture. When the cell density was 80%, the cells were exchanged for culture in DMEM differentiation medium containing 2% pregnant HS (Gibco, Thermo Fisher Scientific) and 1% PS. BSCs were then immunofluorescently stained with an antibody against Pax7 (mouse anti-Pax7,1:200, Proteintech), a key marker factor for BSCs. Finally, qualified Pax+BSCs were stored in liquid nitrogen.
BSCs were inoculated in 6-well plates (approximately 3×104 cells per well), 12-well plates (approximately 1.5×104 cells per well), and 96-well plates (2×103 cells per well) and cultured in DMEM containing 10% FBS and 1% PS in an incubator at 37°C with 5% CO2. Cells were then treated with different concentration gradients (50, 100, 200, 400, 800 μM) of AA for 24, 48 and 72 h. The treated cells were collected for the Cell Counting Kit 8 (CCK-8) assay, 5-ethyl-2′-deoxyuridine (EdU) proliferation assay, RNA extraction, real-time quantitative PCR (RT-qPCR), immunofluorescence assay, and Western blotting.
At the indicated time points, CCK8 assays were performed to analyze the viability of cells. The BSCs (5×103 per well) were passaged into 96-well plates. Different concentration gradients (50, 100, 200, 400, 800 μM) of AA were added from the stock solution, and skeletal muscle satellite cells were treated for 24, 48, and 72 h. After that, the culture solution was discarded from the 96-well plates. Cells were washed with PBS containing 1×PS, 10 μL of CCK-8 solution with 100 μL DMEM was added to each well, and the cell culture plates were incubated at 37°C in a 5% CO2 incubator for 2 h. Absorbance at 450 nm was measured using a microplate reader with six replicates per treatment.
BSCs proliferative activity was detected with the fluorescence imaging assay according to the kit instructions. Briefly, BSCs were propagated on Matrigel-coated 96-well plates (5×103 cells per well) with AA at concentrations of 0, 100, 200, and 400 μM in three replicates per group and incubated for 48 h. Then, cells were incubated with 100 μL at 50 μM concentration of EdU medium for two hours fixed with 4% paraformaldehyde and permeabilized 0.1% Triton X-100. 100 μL of Hoechst reaction solution was added per well, and placed on a shaker for 30 min at room temperature with escape light, and then photographed with a fluorescence microscope.
Integral RNA was extracted from cells incubated by using TRIzol reagent according to the manufacturer’s instructions (Thermo Fisher Scientific). The FastKing One Step kit was used to synthesize complementary DNA, following the manufacturer’s protocol (Tiangen Biotechnology, Beijing, China). SYBR Green kit (SuperReal PreMix Plus; Tiangen Biotechnology) was used to determine the expression of genes with PCR System (Agilent Mx3000/5p; Agilent Technologies, Santa Clara, CA, USA). Denaturation of cDNA for 15 min at 95°C followed by 40 cycles of amplification at 95°C, 60°C, and 72°C for 15, 30 and 30 s, respectively.
Genes involved in the assessment were Pax7, proliferating cell nuclear antigen (PCNA), tumor proliferation antigen (Ki67), nucleoside base transporter protein (SLC23A2), cell cycle protein-dependent kinase 1 (CDK1), cell cycle protein-dependent kinase 2 (CDK2), to identify the regimen of myogenesis in cultured BSCs. Endogenous reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was utilized to standardize the target RNA profiles and the expression of genes was assessed using the relative quantification (2-△△Ct) method (Oppi-Williams et al., 2013); primer sequences used in this study are displayed in Table 1.
Total protein was obtained using a Radio immunoprecipitation Analysis Buffer (Beyotime Biotechnology, Shanghai, China). Protein concentrations were quantified by using a Pierce BCA protein assay kit (Thermo Fisher Scientific). After electrophoresis of the equal amount of protein on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, the protein was transferred onto polyvinylidene fluoride (PVDF) membranes (Merck, Darmstadt, Germany). The transferred membranes were dam up with 5% skim milk for 1 h and thricely washed with 1xTBST (20x-Tris-buffered-saline with 0.1% tween 20; Solarbio) and overnight incubated at 4°C on a shaker with primary antibodies (Pax7, 1:2,000; MyHC, 1:2,000; MyoD, 1:10,000; MyoG, 1:10,000; Akt, 1:2,000; p-Akt, 1:2,000; mTOR, 1:1,000; p-mTOR, 1:1,000; P70S6K, 1:1,000; p-P70S6K, 1:1,000). After overnight incubation washed with 1xTBST and then incubated for one hour at room temperature with the secondary antibody (goat anti-rabbit secondary antibody, 1:5,000; goat anti-mouse secondary antibody 1:500). After washing, the HRP chromogenic solution was evenly dropped onto PVDF membranes, and photographed by an Azure 600 multifunctional imaging analyzer.
BSCs were cultivated in 24-well plates and rinsed three times with PBS (Solarbio), then fixed with 4% paraformaldehyde for 20 min at room temperature; perfused with 0.1% Triton X-100 for 30 min. After that cells were incubated with 5% bovine serum albumin (Solarbio) on a shaker for 1 h. Then cells were incubated with primary antibody against MyHC (1:2,000, Proteintech) overnight at 4°C. After washing with PBS, the cells were incubated with fluorescence-labeled secondary antibodies for 1 h at room temperature. The nuclei were stained with DAPI (4′, 6-diamidino-2-phenylindole, 10 μg/μL, BIOSS) for 15–20 min. A fluorescence microscope (OLYMPUS BX53) was used to photograph images on “cell SensTM Entry software (Olympus, Tokyo, Japan)”.
Data were statistically analyzed using GraphPad Prism (version 6.01) software (GraphPad, San Diego, CA, USA). The differences between the two groups were analyzed using Student’s t-tests, and differences among more than two groups were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni multiple comparison test. The results were presented as the mean value±SD. Differences with a value of p<0.05 were considered statistically significant.
Results
BSCs were treated with different concentrations of AA (50, 100, 200, 400, and 800 μM) for 24, 48, and 72 h, and survival of cells was determined by CCK-8. BSCs showed a significant (p<0.05) increase in cell viability from 24 h to 72 h compared to the control (0 μM). Cell viability was significantly increased (p<0.05) under 100, 200, and 400 μM AA treatments, whereas significantly decreased at 72 h (p<0.05) in the 800 μM AA-treated group (Fig. 1A). Subsequently, after adding different concentrations of AA to treat the BSCs for 48 h, it was observed microscopically that there was an increase in the number of cells, an increase in the density, a good state of cell growth, and some refractoriness under the treatment conditions with AA concentrations of 100 and 200 μM in contrast to the control group (0 μM), whereas there was an insignificant difference in the number of cells under the treatment condition with AA of 400 μM (Fig. 2).
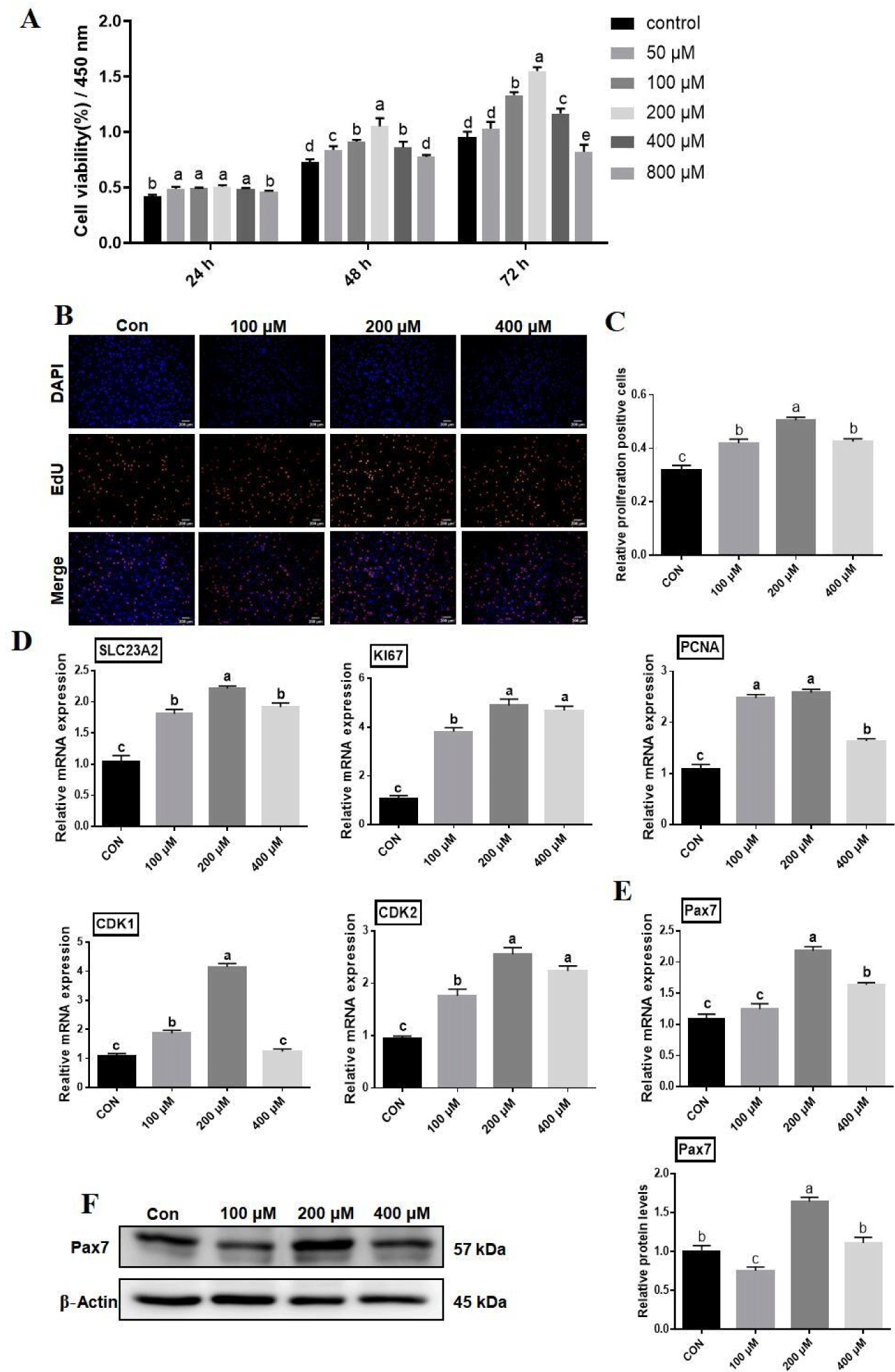
The rate of positive cells was detected by EdU staining under a fluorescence microscope after treating BSCs with different concentrations of AA for 48 h. The rate of EdU-positive cells was extraneously increased (p<0.05) in the AA-treated groups at 100, 200, and 400 μM compared to control (0 μM), whereas there was the inconsequential change in the rate of EdU-positive cells between the AA treated groups at 100 and 400 μM (p>0.05; Fig. 1B).
RT-qPCR analysis showed that mRNA expression of SLC23A2 was significantly increased by different concentrations of AA compared to the control (p<0.05; Fig. 1D), suggesting that BSCs take up AA via Sodium-dependent vitamin C transporter 2 (SVCT2). mRNA expression of CDK2, Ki67, and PCNA in BSCs was significantly increased (p<0.05) under different concentrations of AA treatment compared to control; mRNA expression of CDK1 was significantly increased (p<0.05) at 100 and 200 μM, while at 400 μM there was insignificant change compared to the control group (Fig. 1D). After treatment of BSCs, the expression level of the proliferation marker Pax7 was examined by RT-qPCR and Western blotting. The mRNA expression of Pax7 was significantly higher in 200 and 400 μM AA-treated BSCs compared to 100 μM and control (p<0.05; Fig. 1E). Pax7 protein expression levels were significantly higher in the 200 μM AA-treated group compared to the control group (p<0.05), in contrast, its protein expression levels were significantly lower in the 100 μM group compared to all other groups (p<0.05), and there was no significant difference between the 400 μM group and the control group (p>0.05; Fig. 1F).
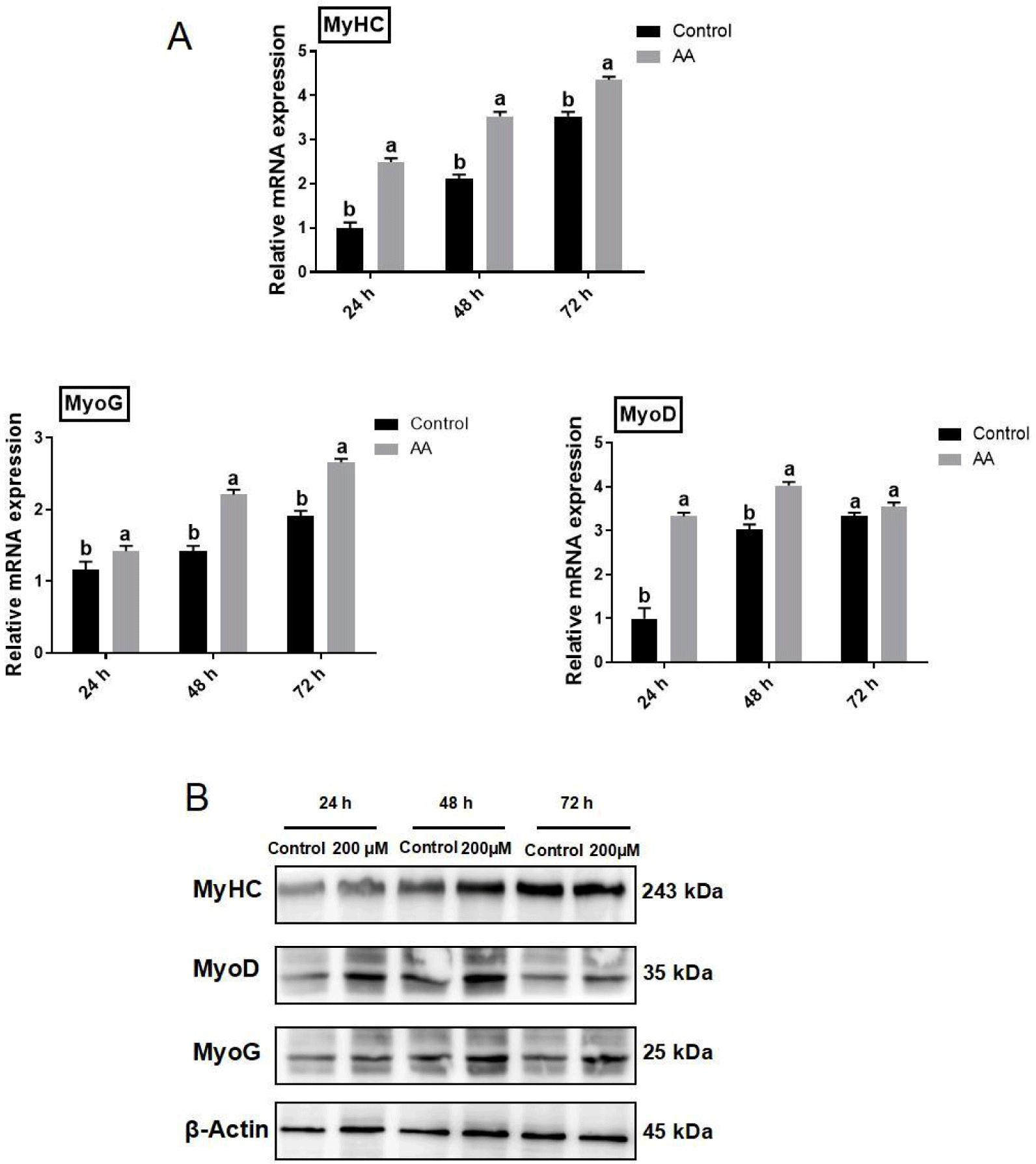
The mRNA expression of both MyHC and MyoG was significantly elevated in BSCs under different concentrations of AA treatment compared to the control group. However, the mRNA expression of MyoD was significantly enhanced under 200 and 400 μM AA treatments (p<0.05), whereas the difference between the control and 100 μM group was insignificant (p>0.05; Fig. 3A).

Western blotting results showed that MyHC protein expression was significantly higher in all AA treated groups compared to the control (p<0.05), whereas difference between 100 and 200 μM (p>0.05) was not significant. Both 200 and 400 μM AA-treated groups increased MyoD protein expression levels compared to controls (p<0.05), while 100 μM-treated groups decreased MyoD protein expression levels (p<0.05). The protein expression level of MyoG was significantly lower in the control group compared to the 200 μM AA treated group (p<0.05), and it was decreased by higher concentration treatment of AA at 400 μM (p<0.05), while there was no significant difference between the 100 μM and the control group (p<0.05; Fig. 3B).
Furthermore, the addition of 200 μM AA-induced BSCs differentiation for 24, 48, and 72 h. Compared with control (0 μM), the AA-treated group significantly increased MyHC and MyoG mRNA expression at all three times (p<0.05), and the expression of MyoD mRNA was significantly elevated in differentiation at 24 and 48 h (p<0.05), and at 72 h of differentiation MyoD mRNA expression was not significantly different compared to control (0 μM; p>0.05; Fig. 4A). To further explore the effect of AA on myogenic differentiation of BSCs, cells were induced to differentiate by the addition of 200 μM AA for 24, 48, and 72 h and analyzed for protein expression. The AA-treated group increased the MyoG protein expression level at all three time periods as compared to the control group (p<0.05), the protein expression level of MyHC was significantly elevated in the 48 and 72 h treatments (p<0.05), and the AA treatment group significantly increased MyoD protein expression level at 24 and 48 h (p<0.05; Fig. 4B).
Cell fusion was detected by immunofluorescence staining under a fluorescence microscope, and the rate of multinucleated myotube fusion was significantly higher in the 200 μM AA treated group compared with the control group (p<0.05; Fig. 3C). Additionally to investigate the effect of AA on the myogenic properties of BSCs, this experiment was carried to see the phenotypic changes after AA treatment of BSCs. An increase in the number of cellular myotubes was observed under the microscope at a concentration of 200 μM of AA inducing differentiation of BSCs for 72 h in comparison to the control group (Fig. 2B).
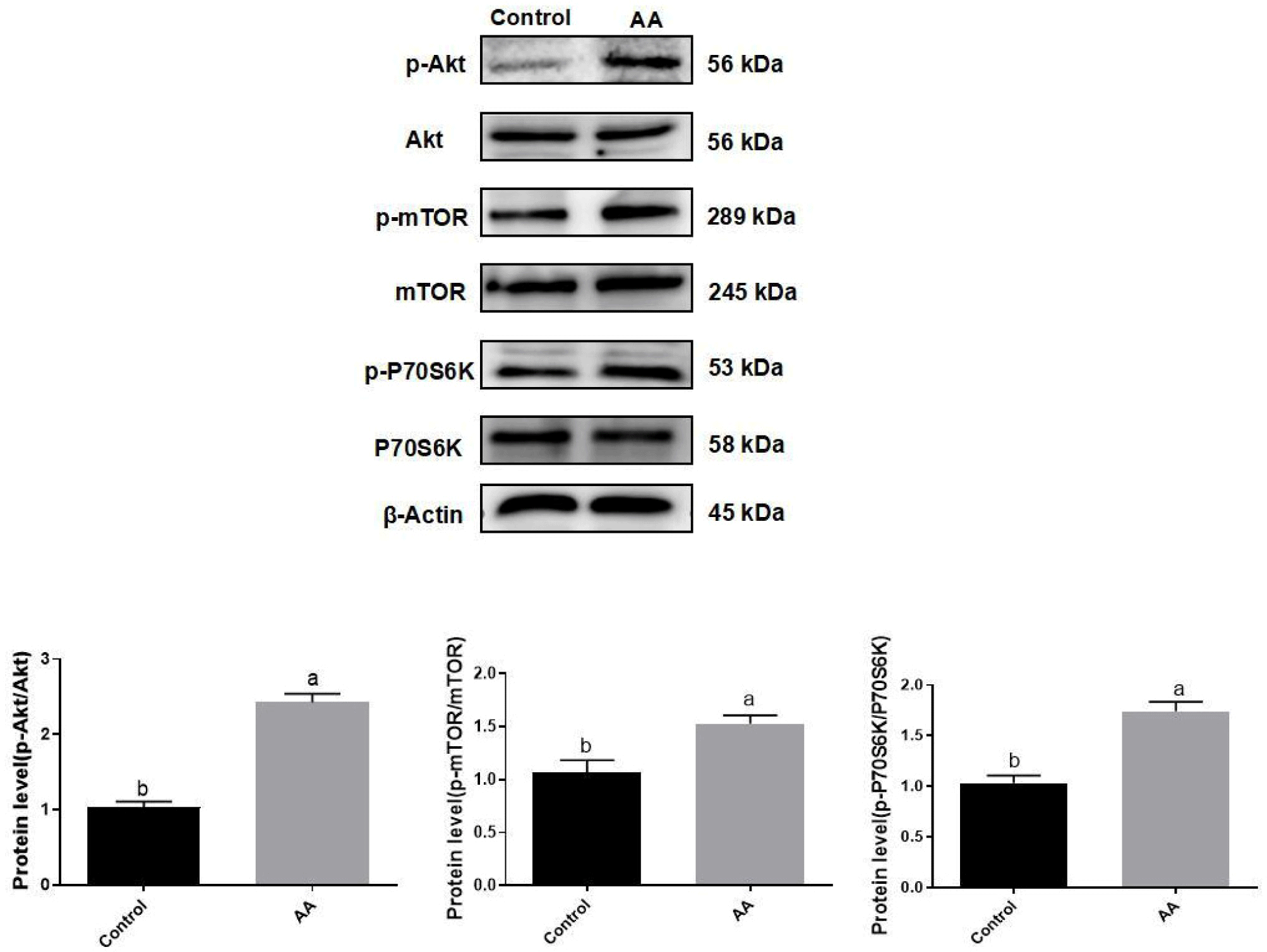
Compared with the control, the expression of p-P70S6K, p-mTOR, and p-Akt was significantly increased in the AA-treated group (p<0.05; Fig. 5), indicating that AA may regulate the proliferation and differentiation of BSCs by activating the Akt/mTOR/P70S6K signaling pathway.
After 48 h treatment of BSCs with various concentrations of rapamycin (10, 20, 100, 200, and 400 μM), viability of cells was measured by CCK-8, which was significantly decreased in the 200 and 400 μM groups compared with the untreated group (p<0.05), while there was no significant variation in cell viability in remaining treatment groups (p>0.05; Fig. 6A), therefore, 100 μM rapamycin was preferred as the final drug treatment concentration for subsequent analyses in this experiment.
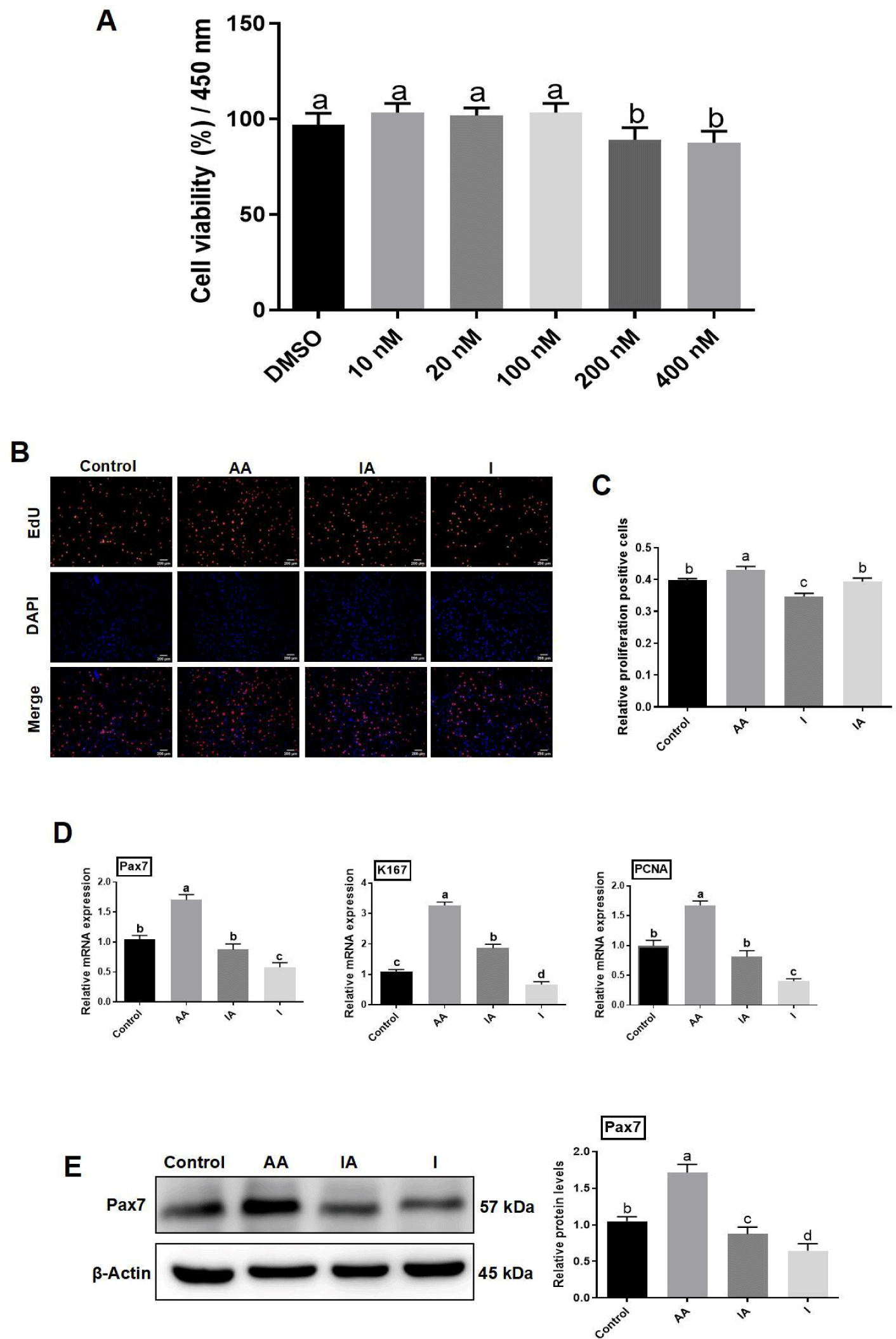
The EdU positive cell rate was significantly decreased in the rapamycin-treated group compared with the control group (p<0.05), and significantly improved in the AA group as compared to the rapamycin and AA co-treatment group (IA; p<0.05; Fig. 6B). Abundance of mRNA of proliferation-related genes was quantified by RT-qPCR analysis and results showed that the mRNA expression of Pax7, Ki67, and PCNA was significantly lower in BSCs in rapamycin treated cells compared with control (p<0.05). Moreover, mRNA expression of Pax7, Ki67, and PCNA was reduced significantly in the IA compared to AA-treated cells (p<0.05; Fig. 6D). Western blotting results showed that compared with control, Pax7 protein expression was significantly reduced in BSCs treated with rapamycin (p<0.05); while significantly declined in IA treatment compared to AA treatment (p<0.05; Fig. 6E).
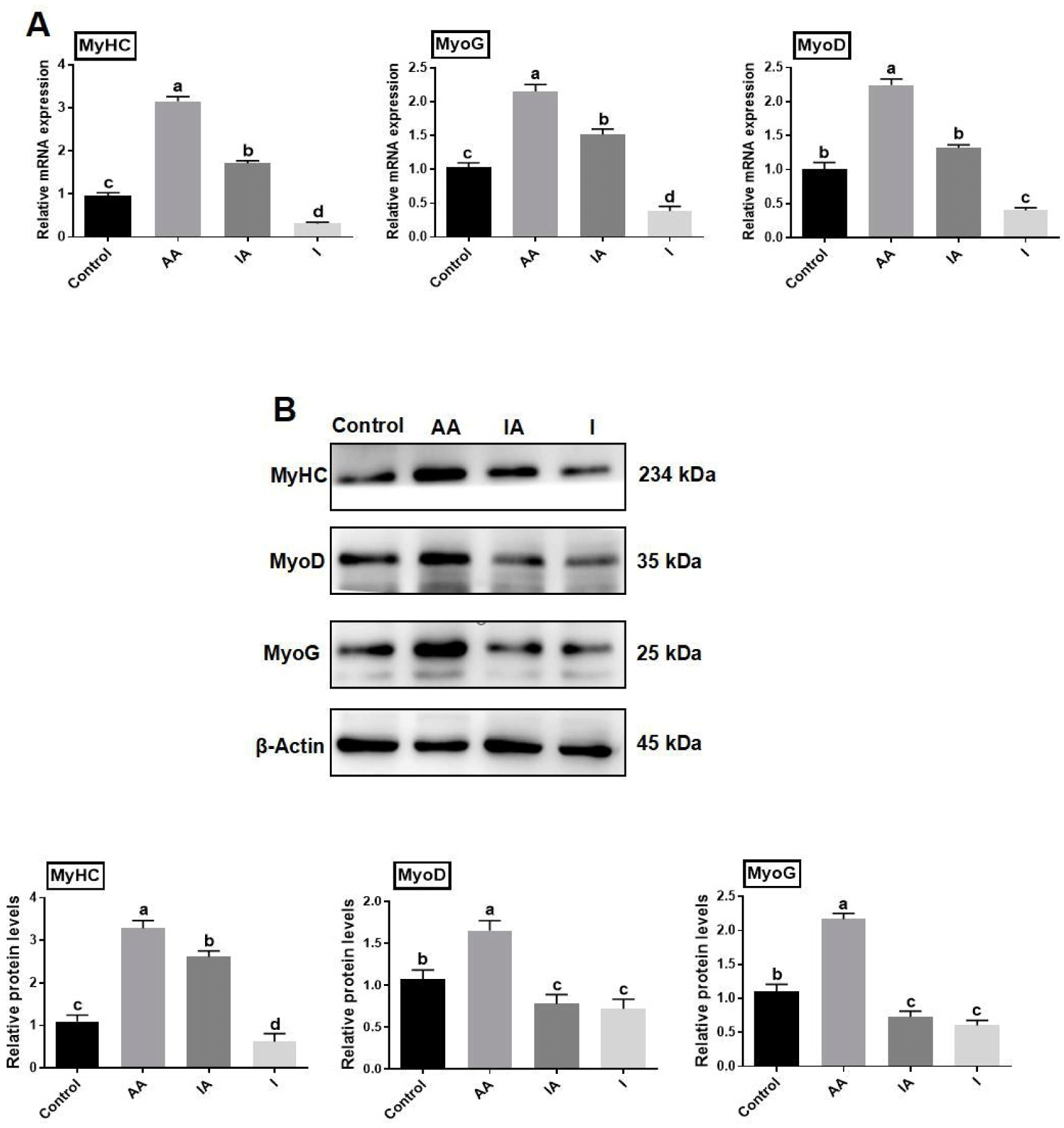
The mRNA expression of differentiation-related genes was explored by RT-qPCR, the rapamycin reduced significantly the expression of mRNA of MyHC, MyoG, and MyoD, compared to the control (p<0.05). Furthermore, the IA treatment significantly decreased the mRNA expression of MyoD, MyHC, and MyoG (p<0.05) compared to the AA-treated group (Fig. 7A). Western blotting assay showed that MyHC, MyoG, and MyoD protein expression was significantly (p<0.05) lower in skeletal muscle satellite cells under rapamycin (group I) treatment compared to control. The rapamycin and ascorbic acid co-treatment group showed insignificant differences in MyoG and MyoD protein expression (p<0.05), while, MyHC was increased significantly in the co-treatment group as compared to rapamycin (p<0.05). Moreover, there was an insignificant difference in MyoG, and MyoD protein expression between rapamycin separately and combined with AA-treated group (p<0.05; Fig. 7B).
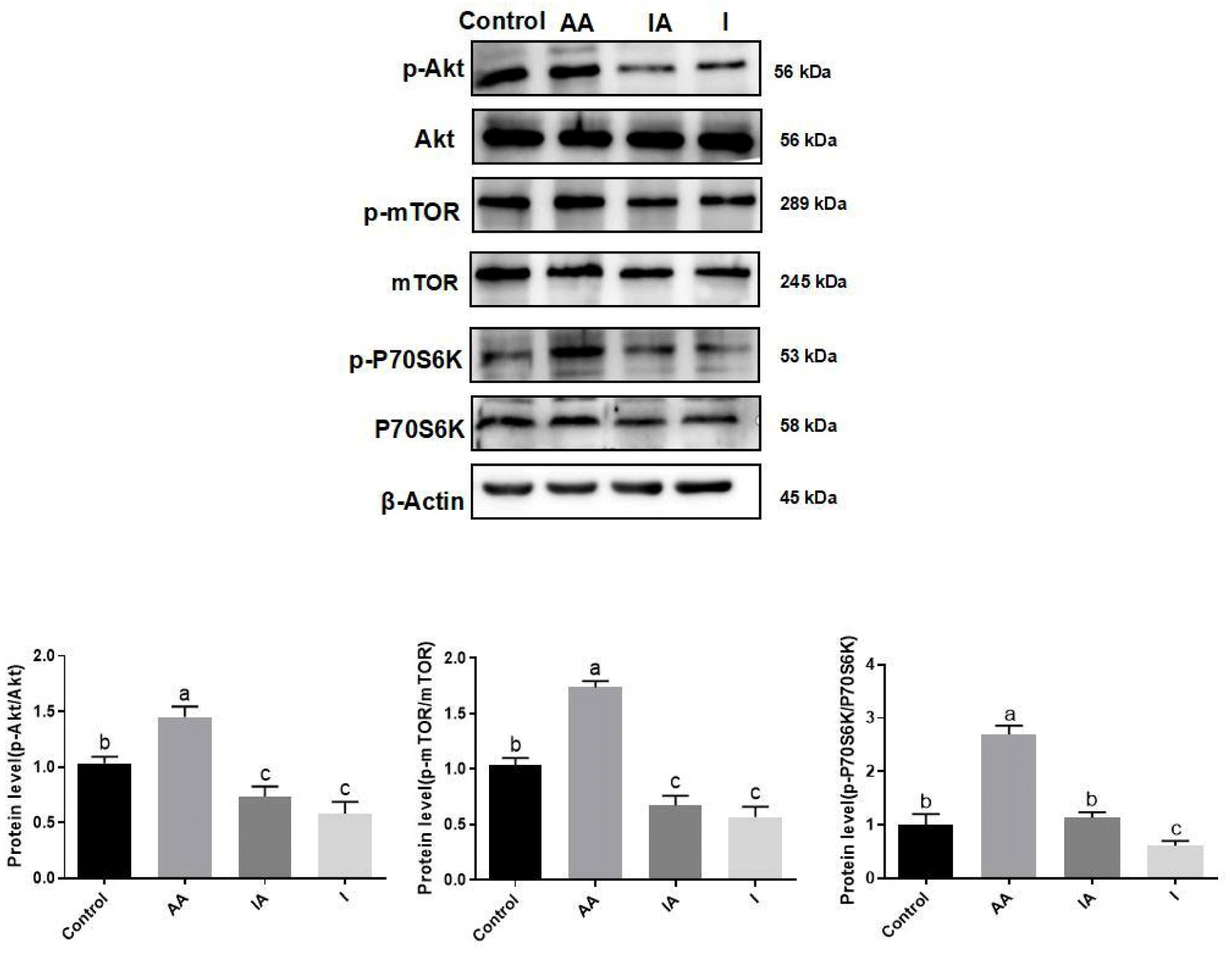
The results of inhibition of the mTOR pathway on BSCs, and protein expression showed a significant reduction in p-Akt, p-mTOR, and p-P70S6K under rapamycin treatment compared to control (p<0.05). In addition, the IA treatment significantly reduced p-Akt, p-mTOR, and p-P70S6K protein expression as compared to the AA treatment (p<0.05; Fig. 8).
Discussion
At the time of its discovery in 1961, the muscle satellite cell was thought to be a satellite cell. These cells are pivotal in muscle regeneration and hold promising prospects for research in cell-cultured meat (Choi et al., 2021; Sousa-Victor et al., 2022). In addition, it was calculated that a cell would require at least 106 expansions to isolate 100 g of cultured meat from 1 g of muscle tissue, gradually losing its function as a muscle stem cell (Li et al., 2022a). In our current research, we demonstrate that AA stimulates the proliferation of BSCs and significantly increases their myogenic potential. AA is an inexpensive food-grade compound that is a good additive for the culture of BSCs and facilitates the industrial production of cultured meat.
The advancing artificial meat technology offers a promising and expanding means of animal protein production. Muscle stem cells are viewed as crucial starting cells for the in vitro production of myofibers, attributed to their capabilities for cellular expansion and differentiation into muscle cells (Daley, 2015). Nevertheless, muscle stem cells lose their ability to function as stem cells over time when cultured continuously, as documented in mice (Fu et al., 2015), humans (Collins, 2006), and domestic animals (Ding et al., 2017). Some intracellular signaling pathways in muscle stem cells are associated with the maintenance of stemness (Charville et al., 2015), in contrast to the maintenance of stemness, some pathways are pushing cells, towards differentiation (Quesenberry et al., 2022) or senescence (Yamakawa et al., 2020). Multiple studies have demonstrated AA’s ability to enhance mammalian cell proliferation, differentiation, and DNA synthesis (Takamizawa et al., 2004; Yu et al., 2014), while also playing a role in cancer therapy (Carr and Cook, 2018). Previous research has demonstrated that AA stimulates the growth of T cells in mice and progenitor cells in humans (Oyarce et al., 2018). However, since AA is unstable and prone to decomposition, AA 2-phosphate (AA2P), a stable derivative of AA, has been chosen as an additive to cell culture media (Bae et al., 2015). In this study, we investigated the effects of AA on the proliferation and differentiation of BSCs. We report here that AA supplementation promotes the proliferation and differentiation of BSCs (Figs. 1 and 3), however, the precise mechanism of how this occurs requires additional investigation. Subsequently, our findings revealed that AA triggers the Akt/mTOR/P70S6K signaling cascade in BSCs, providing fresh insights into AA’s impact on skeletal muscle growth.
One of the primary obstacles in developing artificial meat is producing substantial muscle tissue. A tissue-engineered meat of this size needs a system of blood vessels and circulation to sustain the viability of the inner cell layer (Stout et al., 2022). In the process of creating skeletal muscle tissue, it is necessary for mature myoblasts to undergo proliferation, differentiation, migration, alignment, and fusion in order to develop into multi-nucleated myotubes (Lehka and Rędowicz, 2020). Cell proliferation and differentiation are essential for cell culture meat. Fang et al. (2022) found that AA could activate the PI3K/Akt/mTOR signaling pathway through IGF1 signaling and promote the proliferation of porcine skeletal muscle stem cells. The results of the experiment demonstrated that adding various concentrations of AA to treat BSCs led to a significant increase in cell numbers. Additionally, the EdU staining results indicated that the proportion of EdU-positive cells in the AA-treated group was notably higher compared to the control group (Fig. 1). This aligns with Zhu et al. (2022)’s findings, demonstrating a notable enhancement in both cell viability and quantity following the treatment of porcine skeletal muscle satellite cells with AA (Zhu et al., 2022). Therefore, after we treated with 100, 200, and 400 μM AA for 48 h, it was observed under the microscope that the AA-treated group showed an increase in the number of cells, an increase in the density of cells, a favorable state of cell growth and a certain degree of refractoriness compared to the control group (Fig. 2A). After collecting the cells, the mRNA expression levels of SLC23A2, a gene that encodes cellular transporter protein, and CDK1, CDK2, Ki67, and PCNA, genes related to cell proliferation, were detected by RT-qPCR. In this study, Ki67, PCNA, CDK1, and CDK2 were characterized, upon treatment of BSCs with AA, the mRNA expression of SLC23A2 was notably elevated compared to the controls, implying that BSCs uptake AA through the SVCT2 transporter. The expression of CDK2 and CDK1 mRNAs was notably augmented in the AA-exposed group, pointing to AA’s ability to foster BSCs proliferation via modulation of cell cycle-associated factors. This aligns with Fang et al. (2022)’s finding. In summary, it was verified that AA treatment accelerated the cell cycle and enhanced the proliferation of skeletal muscle satellite cells by different means, both at the DNA level and gene expression level. Mitsumoto et al. (1994) discovered that AA enhanced the production of proteins specific to muscle glucose and ion transport, accelerated collagen synthesis, and promoted differentiation of myoblasts by increasing MyoG expression. Furthermore, AA holds a pivotal position in maintaining cellular equilibrium and functions as a stimulatory agent in cell differentiation processes. Li et al. (2022b) found revealed that AA stimulates the expression of SVCT2 and cysteine-rich protein 3 (CSRP3), thereby advancing C2C12 cell differentiation and muscle repair in mice. Additionally, AA modulates muscle development by facilitating the interplay between CSRP3, MyoD, and MyoG, which enhances myofibroblast differentiation and expedites muscle damage recovery. AA has different effects on different cell types, probably because it exerts different mechanisms of action in different cell types. In this experiment, we constructed an in vitro myogenic differentiation model of BSC and used different concentrations (100, 200, and 400 μM) of AA differentiation treatment for 72 h. A significant increase in the number of myotubes, the myotube fusion rate, and good cellular status of the cells were observed under the microscope (Fig. 2B). Following cell collection, the expression levels of the myogenesis regulators MyoD, MyoG, and MyHC were quantified using reverse transcription RT-qPCR. Upon treatment with AA, the mRNA expression of myogenic differentiation-related genes MyoD, MyoG, and MyHC was notably enhanced, resulting in a corresponding augmentation in protein levels compared to the control group. The concentration that optimizes the differentiation of skeletal muscle satellite cells was determined to be 200 μM. After 24, 48, and 72 h of induction of differentiation using 200 μM AA, the results showed significant up-regulation of mRNA expression and protein expression levels of MyoG and MyHC myogenic differentiation-related genes in a time-dependent manner. The aforementioned results align with Ikeda’s observations, suggesting that AA stimulates the differentiation of BSCs in a dose- and time-dependent fashion, analogous to its effects on C2C12 myogenic cells (Ikeda et al., 2017). The aforementioned findings demonstrate that AA promotes BSC differentiation in a time- and dose-dependent manner, evidenced by alterations in gene expression levels that correspond to changes in the cellular phenotype. The results imply that AA facilitates the differentiation process of skeletal muscle satellite cells in Yanbian cattle, aligning with the conclusions drawn by Li et al. (2022a) foundings. In addition, it has been shown that AA increases the expression level of MyoG protein and promotes the fusion of multinucleated myotubes (Shima et al., 2011). Immunofluorescence analysis demonstrated a substantial elevation in both the cellular myotube count and the rate of myotube fusion following 72-h exposure of BSCs to 200 μM AA, in contrast to the control group (Fig. 3C). In summary, it was found that AA had a significant regulatory effect on the process of myogenic differentiation of BSCs, and promoted the differentiation of BSCs in a time and dose-dependent manner.
Skeletal muscle growth is influenced by a variety of signalling pathways including mTOR, Notch, and Wnt, and the expression of mTOR family members is critical for skeletal muscle development and regeneration. The mTOR signalling pathway regulates cell fate by progressively amplifying extracellular signals through the Akt/mTOR/P70S6K molecular cascade. The Akt/mTOR/P70S6K signaling pathway is not only important for cell survival and self-renewal, but is also crucial for nutrient uptake, intracellular substance synthesis, and metabolism, and can power the synthesis of proteins, lipids, and other substances essential for life activities (Yu and Cui, 2016). Overall, the signaling pathway has a relatively wide range of roles and has a huge effect on stem cell proliferation and differentiation by affecting energy metabolism, protein synthesis and so on. The most critical step in the activation of cellular signaling pathways is protein phosphorylation, which involves the activation or inactivation of proteins. This experiment employed Western Blotting for a quantitative assessment of the phosphorylation levels of signaling pathway proteins. As shown in Fig. 5, the phosphorylation levels of the signalling pathway proteins were increased, and the phosphorylation levels of Akt, mTOR, and P70S6K were elevated about 2.3-fold, 1.5-fold, and 1.8-fold, respectively. The aforementioned findings indicate that AA potentially contributes to enhancing the proliferation and differentiation of BSCs through the activation of the Akt/mTOR/P70S6K signaling cascade.
To deeply investigate the activation of the Akt/mTOR/P70S6K signalling pathway by AA in BSCs, rapamycin, a key protein inhibitor of this signalling pathway, was used to determine the relevance of AA action to this signaling pathway. Rapamycin was first screened for a concentration of 100 μM that did not significantly inhibit cell growth (Fig. 6A). At the same time, 200 μM AA and the corresponding concentration of inhibitor were added to observe whether the proliferative and differentiating effects of AA were reduced, and to confirm the effects of AA on the Akt/mTOR/P70S6K signaling pathway. Upon co-treatment with rapamycin, notable reductions in the mRNA and protein levels of proliferation markers like Pax7, as well as those involved in muscle development and differentiation such as MyoG, were observed, in contrast to the treatment with AA alone (Figs. 6 and 7). Concurrently, the co-administration of AA and rapamycin led to a marked downregulation in the expression of phosphorylated Akt, mTOR, and P70S6k proteins, as compared to the AA-only treated group (Fig. 8). The experimental data further corroborated that AA modulated the proliferative potential, myogenesis, and differentiation of BSCs through the Akt/mTOR/P70S6K signaling cascade. In previous studies, most of them focused only on the antioxidant activity of AA or targeted the molecular mechanisms by which the vitamin regulates collagen synthesis and epigenetic modifications (D’Aniello et al., 2017; Padayatty and Levine, 2016), and have not yet elucidated the intracellular signaling pathways associated with the action of AA. In this study, having observed the stimulatory effects of AA on BSC proliferation and differentiation, we delved into the underlying mechanism and identified the Akt/mTOR/P70S6K signaling pathway as a pivotal regulator in this process. The findings of this research possess significant implications for the subsequent targeted screening of small-molecule compounds or alternative nutrients with the potential to modulate muscle stem cell proliferation and differentiation. The findings of this study offer significant insights for the development of cost-effective, efficient, and animal-free culture systems aimed at cultivating meat products using small molecule compounds or alternative nutrients to regulate muscle stem cell proliferation and differentiation. We are optimistic that advancements in technology will soon mitigate the costs and regulatory hurdles associated with cultivated meat production.
Conclusion
In the current study, we revealed that AA promoted BSCs proliferation over and above upgrade their myogenic potential. The addition of rapamycin significantly inhibited AA-induced BSC proliferation and differentiation. The potential mechanism is through activation of the Akt/mTOR/P70S6K signaling pathway and these effects may be ascribed to accelerated cell cycle concatenation and increased MyoD expression. These findings indicates that AA has the potential to improve the culture system of BSCs in order to enhance cultured meat production more efficient and economical.













