Introduction
Dry-cured ham is a representative processed meat product with a long history and tradition and is produced through dry aging (Gradinarska et al., 2019). During aging, the texture of dry-cured ham varies owing to factors such as temperature, RH, and starter culture conditions, which affect protein degradation, lipid oxidation, and microbial activity (Chen et al., 2021; Sugimoto et al., 2020). Therefore, the selection of a starter culture and the establishment of an appropriate temperature for dry-cured ham are crucial factors influencing the taste characteristics of the product, and it is important to use the desired strain among various microorganisms as a starter culture (Lee et al., 2023).
Debaryomyces hansenii and Penicillium nalgiovense are the dominant microorganisms involved in ham dry-curing (Coton et al., 2021), and the composition of volatile compounds generated during aging influences the sensory characteristics (Li et al., 2021). Also, a mixed starter culture, rather than a single starter culture, enhances the flavor of the product (Wang et al., 2021a), and the same microorganisms that were isolated and identified exhibit different activities (Denkova et al., 2013). Cano-García et al. (2014) reported that when two types of D. hansenii isolated from fermented sausages were inoculated into dry-cured fermented sausages, it affected the volatile compound composition of the final product. Therefore, the inoculation of microorganisms used in the starter culture during dry-cured ham production can significantly influence its flavor (Kim et al., 2022).
The flavor is also influenced by temperature, which impacts chemical and biochemical reactions that affect the final product characteristics (Andrés et al., 2005). Furthermore, relatively high aging temperatures stimulate lipid oxidation and protein breakdown, thereby influencing the rates of compound formation and degradation (Andrés et al., 2004). The optimal temperature for protein degradation in traditionally aged meat products is typically known to be between 15°C–20°C, but for short-term aging, it may be a lower temperature that induces protein degradation (Palhares et al., 2023). In this study, the aging process was initiated at 20°C and 25°C, and various inoculated starters were used by a mixture D. hansenii isolated from Doenjang with D. hansenii and P. nalgiovense isolated from fermented sausage. The sensory properties of the starter cultures were analyzed by examining the general components, salinity, color, shear force, and sensory evaluation of the dry-cured ham. The effect of these starter cultures on flavor was investigated using electronic tongue and nose analyses. In addition, this study represents a step toward confirming the potential of a new starter culture. The produced dry-cured ham considered the variation to be attributed to the starter used and temperature as a variable, and the study proceeded with the assumption that there were no other variables present, except for sameness or the absence of variables.
Materials and Methods
SMFM2021-S8 (D. hansenii) and SMFM2021-S6 (P. nalgiovense) were isolated from fermented sausages produced in Korea. SMFM2021-D1 (D. hansenii) was isolated from Doenjang produced in Korea. The isolated D. hansenii and P. nalgiovense strains were supplied by Yoon BioTech (Seoul, Korea; Lee et al., 2023). The P. nalgiovense Saterkulturen Edelchimmel of commercial strains was supplied by the Almi GmbH (Oftering, Austria). In the microbial cultivation process, isolated microorganisms were mixed with YM Broth and cultured at 25°C. Subsequently, they were separated using a centrifuge (Supra R22, Hanil, Daejeon, Korea; 4°C, 10,923×g, 10 min). Furthermore, to remove the YM Broth, it was diluted with saline solution and subjected to three rounds of centrifugation before utilizing the microorganisms. All yeast and mold were used at 8 Log colony forming unit (CFU) per gram (Log CFU/g). The description of the treatment and starter culture is shown in Table 1.
1) Spice: 0.5% of black pepper, 0.15% of juniper berry, 0.2% of sugar, 0.1% of garlic powder, 0.15% of cilantro seed powder, 1% of nitrite pickling salt, 2.5% of salt, 0.05% of bay leaf.
C, Penicillium nalgiovense Saterkulturen Edelchimmel of commercial starter; DD, Debaryomyces hansenii SMFM2021-D1 of Doenjang produced in Korea and SMFM2021-S6 of P. nalgiovense of fermented sausages; FD, D. hansenii SMFM2021-S8 of fermented sausage produced in Korea and SMFM2021-S6 of P. nalgiovense of fermented sausages; DFD, DD+FD; 20, 20°C; 25, 25°C.
The raw pork used in this study was supplied and used by Gluteus medius (Ihomemeat, Seoul, Korea) 24 h after slaughter, and connective tissues and excessive fat were eliminated from G. medius and used for experiments. Thereafter, a mixture of salt and spices (black pepper 0.5%, juniper berry 0.15%, sugar 0.2%, garlic powder 0.1%, cilantro seed powder 0.15%, nitrite pickling salt 1%, salt 2.5%, bay leaf 0.05%) were applied to the surface of G. medius. The sample was then subjected to a 7-day vacuum-drying process. During the salting period, the sample was flipped once every 24 h. After the salting period, the sample was rinsed with cold water for 2 min. Subsequently, indoors at a temperature of 20±5°C and flipped once every 24 h, undergoing a 2-day drying process. As the aging process began, the RH and temperature gradually decreased from 70% RH and 20°C or 25°C, respectively. Finally, the sample was aged for 6 weeks under conditions of 65% RH and 16°C (for the treatment starting at 20°C) or 65% RH and 21°C (for the treatment starting at 25°C). All samples were collected from three G. medius and all experiments were conducted on the day of sampling.
The moisture and crude protein contents were determined according to the AOAC (2010) method. As for the moisture content, 1 g of the sample was dried for 24 h in a 105°C atmospheric pressure dryer. It was calculated by comparing it with the weight before drying. The crude protein content was measured by Kjeldahl (AOAC 928.08) method.
The samples were subjected to homogenization using an ultra turrax homogenizer (HMZ-20DN, Poonglim Tech, Seongnam, Korea) at a speed of 6,451×g for 1 min, with distilled water (DW) added at a ratio of 1:4. Following the homogenization process, the mixture was determined using a salt meter (SB-2000PRO, HM DIGITAL, Seoul, Korea).
Chromaticity was measured using a pulse xenon lamp calibrated with white standard plates (CIE L*: +97.83, CIE a*: –0.43, CIE b*: +1.98), a 2° standard observer, an 8 mm reading surface area, and a colorimeter (CR-10, Konica Minolta, Osaka, Japan) equipped with a standard light source D65. The chromaticity was measured in the inner cross-section.
The samples were prepared in dimensions of 1×2×1 cm3 and subjected to analysis using a texture analyzer (TA1, Lloyd, Largo, FL, USA). The analysis was conducted under the following conditions: Newton (N) unit of force, test speed of 21.0 mm/s, head speed of 21.0 mm/s, distance of 20.0 mm, and applied force of 5.6 N.
The taste sensor system (Astree V, Alpha MOS, Toulouse, France) was utilized for e-tongue analysis. A 3 g sample was homogenized in 12 mL of DW using a homogenizer (AM-5, Nissei, Tokyo, Japan) at a speed of 6,451×g for 1 min. Following homogenization, it was filtered through filter paper, and the filtrate was diluted 5,000-fold in DW. The e-tongue system recorded signal intensities at each sensor, including CTS (saltiness), NMS (umami), and AHS (sourness), along with auxiliary sensors SCS and CPS, and standard sensors PKS and ANS.
E-nose analysis was performed using the Hercules NEO flash gas chromatography e-nose system (Alpha MOS). Each 5 g sample was placed in a 20 mL head-space vial, which was then sealed. The sealed vial was subjected to stirring at 80°C for 20 min. Subsequently, 5 mL of volatile compounds were collected using an automatic sample collector. These collected volatile compounds were injected into the gas chromatography injection port at a rate of 125 μL/s and a temperature of 200°C. The analysis of these injected volatile compounds was conducted simultaneously using two columns (MXT-5/MXT-1701, Restek, Bellefonte, PA, USA) with a trap absorption temperature of 80°C, a trap desorption temperature of 250°C, and an acquisition time of 110 s. To identify the components, an alkane C6–C16 standard solution was utilized, with the application of Kovat’s index.
The sensory evaluation was conducted in accordance with the guidelines of the Institutional Bioethics Committee of Kongju National University (KNU_IRB_2021-54). Ten trained panels were recruited and sensory evaluation was conducted at a separate location on the same day. The trained panel was an individual with prior experience evaluating meat products. The sample was sliced into a thickness of 0.2 mm and presented. The sensory evaluation included the evaluation of texture, tenderness, off-flavor, flavor, saltiness, sourness, and overall evaluation. Organizational sense was given a higher score as the texture suitable for dry-cured ham was shown, and off-flavor was given a higher score as it was lower. The higher the tenderness, flavor, saltiness, sourness, and overall evaluation, the higher the score was given. Each panel evaluated randomly selected samples using a standardized 10-point scale and was directed to give higher scores to the samples considered most preferred.
At least three repetitive trials were performed to evaluate all the data obtained in this study. Within specific treatment categories, the results of the aging difference test revealed significant distinctions denoted by rows labeled as a, b, and c. Similarly, within specific aging periods, the results of the treatment difference test indicated variations along columns labeled A, B, and C. Consequently, all experimental analyses underwent a one-way ANOVA, evaluating 8 treatments across 4 storage periods (Kim and Kim, 2023). This process was repeated three times, and the data were statistically analyzed using SAS (version 9.4 for Windows, SAS Institute, Cary, NC, USA). Data were analyzed using a one-way analysis of variance, and Duncan’s multiple-range test was used for mean comparison. Significance was determined at p<0.05. The results are presented as mean±SE.
Results and Discussion
Table 2 presents the moisture and crude protein content of sample according to the type of starter and temperature. The moisture content decreased in all treatments as the aging period increased. Under the same starter conditions, a tendency for higher moisture content was observed in the ham aged at 20°C compared to that at 25°C, during all aging periods. At 6 weeks of aging at 25°C, C25 tended a higher moisture content than the other treatments. The crude protein content significantly increased with the aging period (p<0.05). During all aging periods, the treatments that received the same starter showed a higher tendency for crude protein content when aged at 25°C compared with those aged at 20°C. During the aging process of meat products, moisture migrates towards the meat’s surface as a dehydration process occurs (Guo et al., 2021). Early in the drying process, free water is lost, and during the aging period, bound water decreases due to dehydration. The drying rate varies according to drying environmental conditions (Álvarez et al., 2021). Parolari et al. (2016) reported that when dry-cured ham was aged for 12 months at 4°C and 16°C, the high-temperature treatment showed lower values. In addition, moisture and protein contents showed a negative correlation with the aging period (Zhu et al., 2021), and the results of this study conformed to this trend. Deng et al. (2022) investigated the microbial composition of Jinhua ham after aging and reported that NaCl content increased as moisture content decreased, thereby inhibiting the growth of spoilage microorganisms. Therefore, it is considered that aging at 25°C has lower moisture content compared to aging at 20°C, which can increase microbial safety and increase crude protein content.
Table 3 presents the salinity of the sample according to the type of starter and temperature. Salinity significantly increased with the aging period in all treatments (p<0.05). At 6 weeks of aging, C20 and C25 did not show any significant difference (p>0.05); however, the other treatments exhibited significantly lower values when aged at 20°C compared to those aged at 25°C (p<0.05). Furthermore, the FD20 exhibited significantly the lowest salinity value (p<0.05), while DFD25 showed significantly the highest value (p<0.05). Dry-cured ham absorbs salt through salt diffusion as NaCl moves from the exterior to the interior of the muscle (Pinna et al., 2020). Aging products are subject to a negative correlation, with moisture content decreasing and relative salinity increasing through the aging process, and in this study, it is presumed that salinity increased with the aging period (Bou et al., 2022). In addition, at higher aging temperatures, the rate of moisture evaporation increases, resulting in a lower moisture content under the same aging period conditions (Parolari et al., 2016). Therefore, DD25, FD25, and DFD25 (aged at higher temperatures) exhibited a higher salinity tendency than DD20, FD20, and DFD20. Additionally, during maturation, the extent of microbial growth influences the amount of moisture lost (Preetha and Narayanan, 2020). In particular, yeast and mold showed higher growth capacity at 25°C than at 20°C, leading to higher salinity in the 25°C treatments (Kim and Kim, 2023). Recently, with the growing emphasis on low-salt-related research in the maturation meat product industry, treatments with significantly lower salinity contents, such as DD20, FD20, FD25, and DFD20, are considered more suitable than the control (Muñoz-Rosique et al., 2022). This indicates the potential for producing low-salt dry-cured ham depending on the starter culture.
Table 4 presents the color of the sample according to the type of starter and temperature. CIE L* increased in all treatments up to 2 weeks of the aging period, and then, it started to decrease starting from 4 weeks. At 6 weeks of aging, FD25 and DFD25 did not exhibit significant differences compared to C25 (p>0.05). Dehydration results in the formation of a thin moisture layer on the muscle surface, causing light scattering and an increase in CIE L* (Marušić et al., 2011). The subsequent decrease in CIE L* is attributed to muscle fiber contraction, which reduces the ability of muscle fibers to bind moisture, resulting in moisture extrusion and drying (Wang et al., 2022). Lorenzo and Carballo (2015) reported a positive correlation between moisture content and CIE L* values during the aging period in dry-cured foal salchichón. The results of this study followed a similar pattern. The CIE a* values showed an increasing trend for all treatments as the aging period increased. At 6 weeks of aging, DD20 and DFD20 did not exhibit significant differences compared to C20 (p>0.05). Similarly, DD25 and FD25 did not show significant differences compared with C25 at this time point (p>0.05). Furthermore, excluding FD20 and FD25, those inoculated with the same starter showed significantly lower CIE a* values under aging temperature of 20°C than at 25°C (p<0.05). Increased salt content promotes the synthesis of nitrosomyoglobin and heme pigments that cause CIE a* (Fernández-López et al., 2007). Therefore, those aged at 25°C exhibited a higher evaporation rate of moisture compared to those aged at 20°C, leading to a higher CIE a* value. CIE b* in all treatments showed an increase at 0 weeks to 2 weeks and then decreased. As for the CIE b* values, there was no significant difference from C in FD and DFD selectors with the same temperature conditions at 6 weeks of aging (p>0.05). Additionally, treatments that received the same starter cultures showed significantly lower values at 25°C compared to 20°C (p<0.05). Oxymyoglobin, which contributes to CIE b*, decreases because the microorganisms inoculated into dry-cured ham consume oxygen (Lorenzo et al., 2012). Furthermore, the rate of lipid and protein oxidation is positively correlated with CIE b*, and it is assumed that the treatments at 25°C showed higher CIE b* values than at 20°C (Wang et al., 2021b). Therefore, it is inferred that the increase in CIE b* values is attributed to the initial oxidation of lipids and proteins during the maturation process, followed by rapid consumption of oxymyoglobin by microorganisms, leading to a subsequent decrease. Finally, it was concluded that FD25 did not show differences from the commercially used C25, and it did not affect the final product color, making it the most suitable starter.
Table 5 presents the shear force of the sample according to the type of starter and temperature. Shear force exhibited an increasing trend with aging in all treatments. At 6 weeks of maturation, compared to C20, DD20 and DFD20 did not show significant differences (p>0.05), whereas FD20 exhibited significantly higher values (p<0.05). However, at 6 weeks of maturation, C25 did not significantly differ from DD25, FD25, and DFD25 (p>0.05). Additionally, samples treated with the same type of starter, excluding starter C, did not exhibit significant differences between temperature conditions (p>0.05). Muscle contraction during the drying process can influence its shear force values (Qu et al., 2020). Furthermore, during the aging period, there has been a negative correlation between shear force and decreasing moisture content, indicating that as moisture content decreases, shear force also decreases (Marušić Radovčić et al., 2016). Increased microorganisms can reduce surface exposure and mitigate moisture evaporation (Zadravec et al., 2020). In previous studies, the microbial growth rate of the treatment was faster than control, resulting in similar values between treatments (Kim and Kim, 2023). Therefore, it is suggested that the same starter, excluding the control, was less affected by temperature conditions, showing no significant differences between aging at 20°C and 25°C. Currently, the shear force values of dry-cured ham available in the market range from approximately to 9–11 (Nam et al., 2018). All the treatments in this study exhibited lower shear force values, suggesting a softer texture. Ultimately, consumers tended to prefer products with a soft texture (Resano et al., 2010). Therefore, the starters used in this study can produce products with shear force values lower than those of commercially available dry-cured ham, catering to consumer preferences for softer textures.
Electronic tongue analysis results of the sample based on the starter and temperature are presented in Fig. 1A, which illustrates the principal component analysis (PCA). Fig. 1B depicts the ranking scores for taste attributes, including umami, saltiness, and richness. Electronic tongue PCA analysis accounted for 97.907% of the total variance, with PC1 (84.529%) and PC2 (13.378%). Notably, most differentiation was driven by PC2. However, the difference between the treatments was not prominent based on PC1. This aligns with the findings of Jiang et al. (2021), who reported that it is challenging to distinguish taste differences in dry-cured ham based on PCA analysis, mainly because the taste of dry-cured ham is significantly influenced by NaCl due to a similar salting process. Hence, distinguishing based on PC1 is considered difficult (Wang et al., 2023). Saltiness varied in terms of ranking scores. Specifically, for ham aged at 20°C, the treatments (DFD20, DD20, and FD20) displayed lower intensity of saltiness compared to the control. In contrast, for ham aged at 25°C, the treatments (DD25, FD25, and DFD25) showed higher saltiness intensities relative to the control. Moreover, excluding C20, saltiness aged at 20°C generally exhibited lower than 25°C. At 25°C aligns with the faster moisture evaporation occurring at higher maturation temperatures (Li et al., 2012). Relatively rapid moisture loss leads to an increase in NaCl concentration and is consistent with the salinity results of this study. The intensity of the sour taste varied in this study. Specifically, for aging at 20°C, the treatments (DFD20, DD20, and FD20) showed higher sour taste intensities compared to the control. Conversely, for aging at 25°C, the treatments (FD25, DD25, and DFD25) exhibited lower sour taste intensity relative to the control. Notably, FD20 had the highest sour taste intensity, whereas DFD25 had the lowest. The umami taste intensity exhibited variations in this study. Specifically, for aging at 20°C, the treatments (DFD20, FD20, DD20) showed higher umami taste intensity compared to the C20. Conversely, for aging at 25°C, the treatments (DFD25, FD25) exhibited higher umami taste intensity, but DD25 showed a lower intensity compared to the C25. The umami taste is enhanced when microbial growth is faster, as proteolysis into umami amino acids and flavor-active peptides (Gallego et al., 2022). Zhou et al. (2020a) reported that when investigating the effect of NaCl concentration of dry-cured ham on taste, the concentration of umami free amino acid (FAA) formed by proteolysis increased during the aging period and the concentration of FAA, which shows relatively sour taste, was low. In conclusion, the aging at 25°C exhibited a lower umami taste compared to those aged at 20°C. This suggests the potential for producing dry-cured ham with reduced distinctive sourness and enhanced umami, depending on the type of starter. Furthermore, it is worth noting that higher scores for umami are associated with increased consumer preference and enhanced flavor in meat products. Therefore, FD25 and DFD25, which showed a tendency towards higher scores, were considered suitable (Dermiki et al., 2013).

Electronic nose analysis results of the sample concerning the starter and temperature are shown in Fig. 2 displaying the PCA, and compound contents in Table 6. Table 6 presents the key compounds that influence the flavor of sample (Ramírez and Cava, 2007; Shi et al., 2019). The electronic-nose PCA showed differences based on the aging temperature, primarily along PC1 (42.827%). Furthermore, along PC2 (27.305%), the aging treatments at 25°C displayed distinct results compared to the control. E-nose compounds showed ethanol, propane-2-one, hexane, 2,4,5-trimethylthiazole, and limonene. The ethanol contents in C25 showed the highest value (p<0.05), whereas DD25 and FD25 exhibited significantly lower values than DD20 and FD20, respectively (p<0.05). Ethanol has a high flavor threshold, suggesting that it has minimal impact on flavor (Ying et al., 2016). When analyzing compounds in Iberian ham, Martínez-Onandi et al. (2017) reported that lower ethanol content was associated with lower water activity, which aligns with the findings of this study. The propan-2-one contents of DD20, FD20, and DFD20 exhibited significantly higher values than those of DD25, FD25, and DFD25, respectively (p<0.05). Propan-2-one belongs to the group of ketone compounds and is associated with a cheesy odor; however, high levels of this compound can negatively affect the quality (Pastorelli et al., 2003). The hexane contents that aged at 25°C exhibited significantly higher values than those aged at 20°C (p<0.05). Hexane can enhance the development of fermented flavors at higher concentrations, thereby improving the quality (Shi et al., 2019). The 2,4,5-trimethylthiazole and limonene contents in C25, DD25, and DFD25 were significantly lower values than those in C20, DD20, and DFD20 (p<0.05), respectively. 2,4,5-Trimethylthiazole compound positively correlates with Staphylococcus, contributing to flavor development (Kim et al., 2022). Limonene is associated with the perception of sourness in meat products, and when its concentration is higher, consumers tend to prefer it less (Pham et al., 2008). In conclusion, DFD25 exhibited significantly lower values for the propan-2-one and limonene peaks, which may be favorable for consumer preference, whereas hexane values were significantly higher than the other treatments, suggesting a potentially better flavor profile.
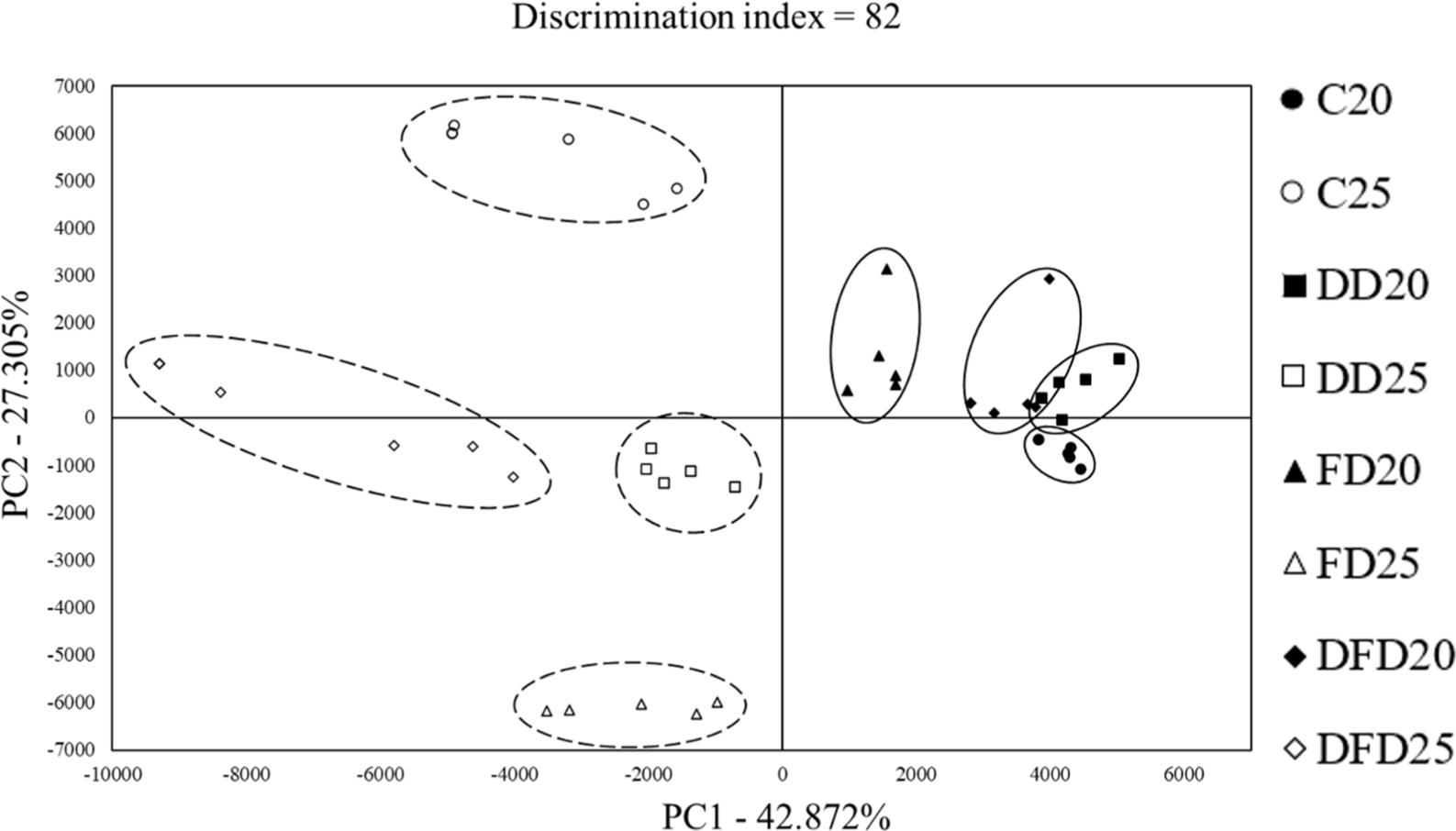
Table 7 presents the sensory evaluation of the sample according to the type of starter and temperature. In terms of texture, DFD25 and DD25 received significantly higher scores than C25 (p<0.05). Regarding tenderness, samples aged at 20°C did not exhibit significant differences among themselves (p>0.05). However, DD25 received significantly higher scores than C25 and FD25 (p<0.05). In the case of off-flavors, C20, C25, FD20, FD25, and DFD25 did not show significant differences among themselves (p>0.05). Resano et al. (2010) conducted a study on consumer acceptability of dry-cured ham and found that consumers prefer tenderness and report negative results associated with off-flavors such as moldy odors. In this study, the treatments that exhibited lower shear force values tended to receive higher scores, and the off-flavor was attributed to the smell of fermentation generated by microorganisms during maturation. In terms of flavor, the DD25 received significantly lower scores than the other treatments (p<0.05), whereas the FD25 showed a tendency toward higher scores. Sugimoto et al. (2020) conducted sensory evaluations of dry-cured ham during aging and reported that treatments with a strong umami flavor resulted in a higher consumer preference. This aligns with the trends observed in the umami taste results of this study. In terms of saltiness, with the exception of the C starter, aging at 25°C in showed a tendency for higher scores. Additionally, the DFD25 received significantly higher scores than DFD20 (p<0.05). Sourness tended to have a higher score in the FD20, whereas the DFD25 showed a tendency toward lower scores. The sensory evaluation and taste results in this study indicated a similarity between saltiness and sourness. This can be attributed to the higher aging temperature at 25°C, resulting in a stronger perception of saltiness compared to aging at 20°C. Sourness can be influenced by the presence of volatile compounds, particularly limonene. This compound is considered a major factor that reduces consumer preference (Zhou et al., 2020b). In conclusion, the overall preference scores showed that relatively low sourness scores, such as those for DD25, FD25, and DFD25, tended to receive higher overall preference scores compared to DD20, FD20, and DFD20. This suggests that sourness has an impact on the overall preference. However, it is deemed necessary to compare the dry-cured ham produced in this study with commercially available dry-cured ham.
Conclusion
This study investigated the influence of dry-cured ham inoculated D. hansenii and P. nalgiovense on sensory characteristics. In all treatments, CIE L* increased until the 2 weeks of aging and then decreased. After 6 weeks of aging, DFD25 showed significantly higher saltiness and CIE a* than C25 (p<0.05). In the electronic tongue analysis, the ranking results indicated that DFD25 exhibited higher saltiness and umami than C25 and lower sourness than the other treatments. In the electronic nose, DFD25 showed a significantly higher hexane content (p<0.05) and significantly lower limonene content than those of the other treatments (p<0.05). The sensory evaluation results indicated that DFD25 showed significantly higher texture scores than C25 (p<0.05) and did not show significant differences in other attributes (p>0.05). Therefore, the DFD25 starter, composed of D. hansenii isolated from fermented sausage and Doenjang, and P. nalgiovense isolated from fermented sausage, which has higher CIE a*, umami, and hexane content and lower sour taste than the control group, was judged to be the most favorable option. However it is judged that optimal environmental conditions should be established through detailed analysis of other climatic conditions as well as aging temperatures in the future.













