Introduction
Freezing meat enables long-term storage and distribution, inhibits the growth of microbes, and minimizes alterations in quality (Leygonie et al., 2012). However, thawing frozen meat leads to adverse alterations in quality, such as excessive exudation of meat juice, discoloration, and accelerated oxidation of proteins and lipids (Kim et al., 2013; Zhang et al., 2017).
Persistent academic and industrial initiatives aim to develop technologies to mitigate adverse alterations in meat quality by minimizing physicochemical changes during the freeze–thaw process (Zhang et al., 2023). Various physical and chemical phenomena that occur in meat during freezing or freeze-thaw processes have been observed, and their effects on meat quality characteristics have been assessed (Leygonie et al., 2012).
Additionally, methods such as controlling freezing and thawing speed, brine injection, vitamin E treatment, high-pressure, ohmic and electrostatic field treatments, and aging technology have been introduced to enhance frozen-thawed meat quality (Cevik and Icier, 2020; He et al., 2014; Hou et al., 2020; Kim et al., 2018; Zhu et al., 2004). Numerous studies have summarized and reviewed the technologies developed over the last two decades (Zhang et al., 2023). Most of these studies have focused on freeze-thaw technology, a widely applied method, regardless of the livestock species and muscle type. However, meat exhibits characteristics (proximate components, pH, and muscle fiber composition) unique to each muscle and displays varying physicochemical characteristics before freeze-thawing (Park et al., 2022). Therefore, these inherent variations in characteristics are anticipated to change depending on the muscle used during meat processing, including freezing and thawing.
However, previous studies have not adequately assessed the variations in muscle or meat cut characteristic-based freezing susceptibility and its influencing factors. Therefore, this review summarized the research results regarding the physical and chemical alterations in significant muscles owing to freezing or freeze-thawing. Moreover, we emphasize the necessity of developing technology to minimize adverse alterations in meat quality owing to freeze-thawing, depending on the characteristics of each muscle. To achieve this, we assessed previous studies related to the freezing and freeze-thawing of meat, specifically focusing on the results of muscles or comparative studies between various muscles or cuts.
Formation of Ice Crystals through Freezing and Its Effect on Muscle Tissues
Freezing meat effectively prevents spoilage by inhibiting the growth of microorganisms (Coombs et al., 2017). However, thawing meat following freezing gradually eliminates its latent heat, resulting in altered physicochemical attributes, including reduced juiciness and water-holding capacity (WHC), discoloration, increased rancidity, and texture alterations (Cheng et al., 2020; Cheng et al., 2021; Park et al., 2012). This is because of the effects of ice crystals that form between and within myofibrils during the freezing process (Dang et al., 2021; Schudel et al., 2021). Meat contains approximately 75% water depending on the species, muscle type, and fat content (Huff-Lonergan and Lonergan, 2005). Among the different water types (bound, entrapped, and free) within meat, the entrapped and free water, crucial for chemical and biochemical reactions in meat, are susceptible to freezing due to ice crystal formation; however, bound water remains non-frozen in meat. Approximately 88% of the total water content in meat is freezable (Xanthakis et al., 2013).
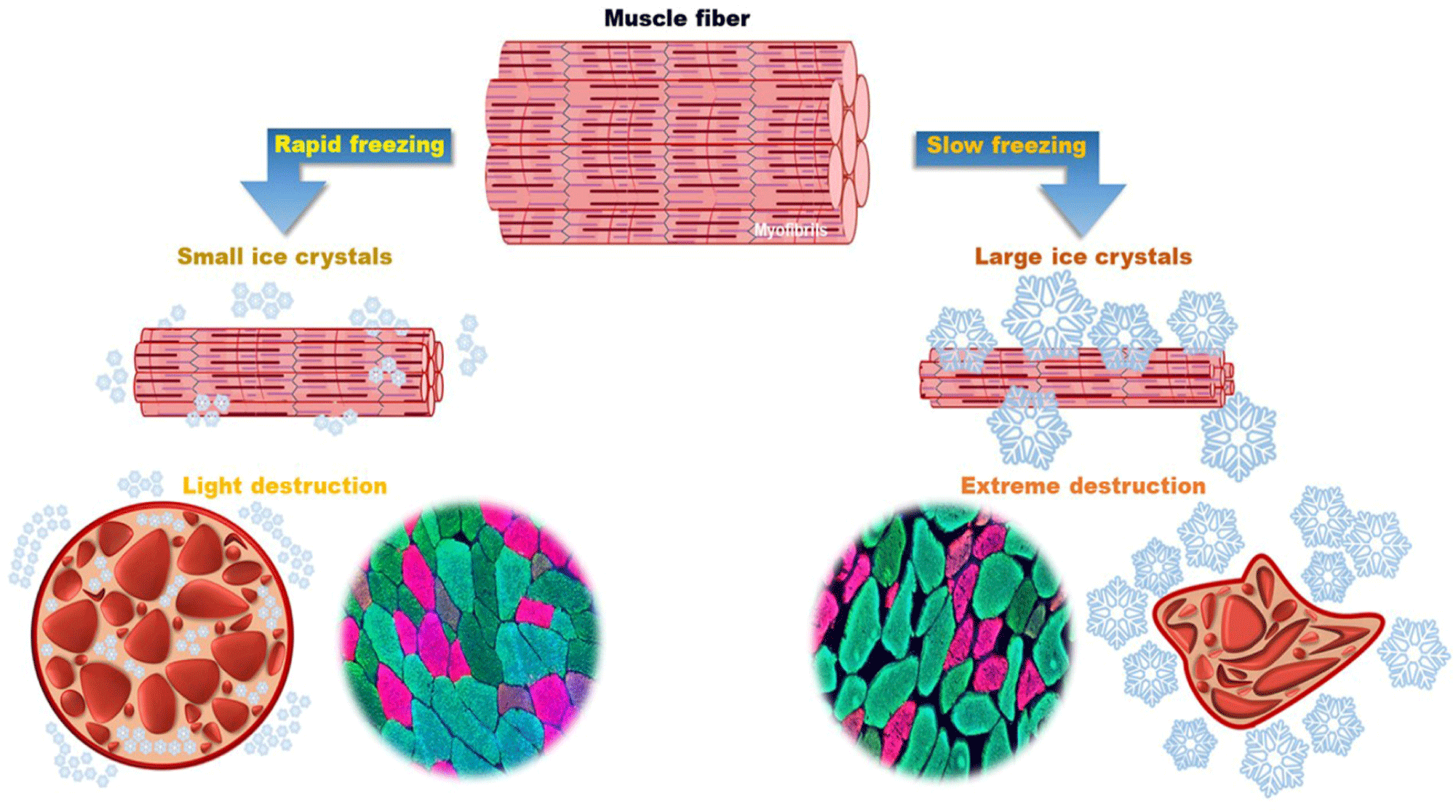
Ice crystal formation in meat typically begins at approximately –1°C, with approximately 75% of the moisture of the forming ice crystals at –5°C (Cooke and Wien, 1971; Huff-Lonergan and Lonergan, 2005). When the core temperature of the meat drops to –12°C, approximately 92% of the moisture forms ice crystals, and the residual moisture maintains the ingredients in the meat, including proteins, in an unfrozen state (Cooke and Wien, 1971; Huff-Lonergan and Lonergan, 2005). While meat is frozen, water initially creates ice crystal nuclei both inside and outside the muscle fibers (myocytes), which then gradually expand in the form of branches, producing large or small crystals (Xanthakis et al., 2013). These ice crystals can physically damage muscle microstructure, resulting in Z-line destruction, I-band weakening, and increased intermyofibrillar space (Añón and Calvelo, 1980; Cheng et al., 2020). The degree of physical destruction within the muscle varies depending on the size of ice crystals (Dang et al., 2021; Schudel et al., 2021).
The freezing rate of meat determines the size and number of ice crystals, which affect the muscle fiber structure (Fig. 1). Rapid freezing leads to the formation of smaller ice crystals outside muscle fibers, whereas slow freezing leads to the formation of larger ice crystals (Cheftel et al., 2002; Fernández et al., 2006; Su et al., 2014). The freezing rate is linked to the heat transfer rate from the outside to core of the meat and is closely related to the uniformity and non-uniformity of the histological and physicochemical properties of frozen or frozen-thawed meat (Choi et al., 2016; Sanz et al., 1999). Drip loss inevitably occurs when the frozen meat is thawed. During thawing, ice crystal formation varies based on the freezing speed of meat, which determines the degree of muscle tissue destruction and drip loss (Dang et al., 2021; Schudel et al., 2021). Additionally, the formation of ice crystals alters the solute concentration both within and outside the muscle fiber, resulting in an imbalance, thereby leading to biochemical and physical alterations in multiple meat components (Bevilacqua et al., 1979; Huff-Lonergan and Lonergan, 2005; Love and Haraldsson, 1961). Therefore, ice crystal formation and physicochemical alterations within the muscle tissue owing to freezing are significant factors that directly or indirectly affect muscle food quality characteristics such as WHC, meat color, flavor, and texture (Cheng et al., 2020; Cheng et al., 2021; Dang et al., 2021; Wang et al., 2020).
Effects of Freezing and Freeze-Thawing on Meat Physicochemical Properties
Freezing is an effective method to extend the shelf life of meat. However, the freezing and thawing processes have adverse effects on meat quality characteristics, such as discoloration, reduction in WHC, and alterations in sensory properties (flavor, taste, and texture; Dang et al., 2021; Wang et al., 2020). A detailed overview of the alteration in color, WHC, texture, and shelf life of meat owing to freezing and freeze-thaw cycles is described below.
The shelf life of meat is usually determined by its appearance, color, flavor, nutritional value, and microbial activity. The various ingredients of meat are crucial factors that affect its quality characteristics and alterations (McMillin, 2008). The moisture of meat is primarily retained in the interstices between the thick and thin myofibril filaments. This occurs within and between myofibrils, and a small amount of water within the muscle is retained through the electrostatic attraction between proteins (Bond et al., 2004; Cheng and Sun, 2008). Additionally, the fat present in meat contains water, which contributes to maintaining its moisture content (Joo et al., 2002). When meat releases moisture naturally or owing to certain factors (cooking, freeze-thawing, and pressing), the exudate contains nutrients and flavor components, such as vitamins, minerals, and amino acids (Añón and Calvelo, 1980; Leygonie et al., 2012; Ngapo et al., 1999). Therefore, WHC (the ability of meat to retain moisture) is synonymous with the effectiveness of retaining various useful ingredients in meat. Additionally, WHC is commonly assessed by measuring the degree of drip loss, thawing, cooking, and purging (Honikel, 1998; Honikel and Hamm, 1994; Huff-Lonergan and Lonergan, 2005).
Freezing significantly reduces the WHC of meat. Thawing of the frozen meat results in excessive exudation of its juices (Leygonie et al., 2012). Therefore, basic and applied studies were conducted to minimize the amount of meat exudate, as listed in Table 1. The primary results of previous studies on the loss of exudates and alterations in WHC in meat based on freezing or freeze-thaw processes are summarized below.
| Species | Muscles | Freezing/thawing/storage conditions | Outcome | References |
|---|---|---|---|---|
| Porcine | M. longissimus dorsi (LTL) | Sequence of freezing and aging: Freezing without aging (FT), aging prior to freezing (AFT), and freezing and aging (FTA) Slow-freezing: –20°C for 8 weeks and thawing 1°C for 2 days Fast-freezing: −80 °C and stored −20°C for 8 weeks and thawing 1°C for 2 days Aging: 1°C for 19 days |
pH: FTA≤FT<AFT ↓ WBSF in FTA with slow-freezing ↑ Purge/thaw loss in FTA unrelated with freeze rate ↑ CIE L* in FTA with slow-freezing ↓ CIE a* in slow-freezing ↓ CIE b* in FTA with slow-freezing |
Kim et al. (2018) |
| Porcine | M. longissimus dorsi M. psoas major (PM) M. semimembranosus (SM) M. semitendinosus (ST) |
Fresh: 4°C for 7 days Freeze-thawing: −20°C for 5 days and thawing at 4°C for 2 days |
- pH in all muscle cut and freeze-thawing - Purge loss ↓ CIE L* in LTL and ST ↑ CIE a* in LTL and SM ↑ CIE b* in LTL and ↓ in PM and ST ↑ WBSF in SM and ST ↑ Cooking loss in LTL ↓ Drip loss in SM and ST ↓ WHC and toughness in SM and ST ↑ Muscle type I and IIA size in ST ↑ Muscle type IIXB amount in SM |
Cheng et al. (2021) |
| Bovine | M. longissimus thoracis et lumborum | Fresh: Aging at 4°C for 2, 7, and 14 days Freeze-thawing: Aging for 2, 7, and 14 days at 4°C, freezing at −20°C for 2 months, and overnight thawing at 4°C |
↓ WBSF over time ↓ WHC in freeze-thawing ↑ Tenderness, juiciness and meat taste in fresh - Panel preference |
Lagerstedt et al. (2008) |
| Bovine | M. longissimus thoracis et lumborum (LT) M. psoas major M. semimembranosus M. semitendinosus |
Fresh: 4°C for 5 days Freeze-thawing: −20°C for 3 days and thawing at 2°C for 2 days |
- pH in all muscle cut and freeze-thawing ↑ CIE L* in LT with freeze-thawing ↓ CIE a* in LT, ↑ CIE a* in SM and ST with freeze-thawing ↓ CIE b* in SM with freeze-thawing ↓ WBSF in LT with freeze-thawing ↑ Purge losses in LT, SM and ST with freeze-thawing ↓ Drip loss in all cuts except PM with freeze-thawing ↓ Cooking loss in LT and PM, ↑ cooking loss in SM with freeze-thawing ↑ WHC and ↓ discoloration in PM than SM ↑ Muscle type I amount in PM than SM |
Cheng et al. (2020) |
| Porcine Chicken | Porcine neck Chicken leg |
Freeze-thawing: Frozen at –18°C, –50°C, and –60°C for 0.5, 1, 2, 3, 4, 5, and 6 months and then thawed at 2°C | ↓ Thawing loss at –60°C, ↑ thawing loss at –18°C during 6 months in porcine neck and chicken leg with freeze-thawing - Cooking loss in pork neck and chicken leg with different temperature ↑ CIE L*, CIE a*, and CIE b* in pork neck frozen at –18°C |
Kim et al. (2020) |
| Bovine | M. longissimus thoracis et lumborum | A4: Ageing at −1.5°C for 4 weeks A4F2: Ageing at −1.5°C for 4 weeks and freezing at −18°C for 2 weeks with fast freezing in freezing solution or slow freezing in air-blast at −18°C A3F2: Ageing at −1.5°C for 3 weeks and freezing at −18°C for 2 weeks with fast freezing in freezing solution or slow freezing in air-blast at −18°C F2: Fast freezing in freezing solution or slow freezing in air-blast at −18°C for 2 weeks |
- pH, cooking loss, drip loss, purge loss and WBSF by freeze rate pH: A4F2>F2>A4>A3F2 Purge loss and drip loss: F2>A3F2>A4F2>A4 Cooking loss: A4>A4F2>A3F2>F2 WBSF: F2>A4>A3F2=A4F2 CIE L*, CIE a*, and C: A4>A4F2, A3F2, and F2 Hue angle: A4<A4F2, A3F2, and F2 |
Kim et al. (2015) |
| Bovine | Hind legs | A: Freezing at −18°C with freezing fluctuation cycles repeat for 3 days B: Freezing at −18°C and −17°C; stored at −18°C for 4 hours, raised to −17°C, then returned to −18°C for another 4 hours with freezing fluctuation cycles repeat for 3 days C: Freezing at −18°C and −15°C; stored at −18°C for 4 hours, raised to −15°C, then returned to −18°C for another 4 hours with freezing fluctuation cycles repeat for 3 days D: Cycled between −18°C and −13°C; stored at −18°C for 4 hours, raised to −13°C, then returned to −18°C for another 4 hours with freezing fluctuation cycles repeat for 3 days |
Free drip loss and cooking loss: A<B<C<D Centrifuge drip loss: A=B<C<D CIE L*: A>C>B>D CIE a*: A>B>C>D CIE b*: A<B<C<D |
Wang et al. (2020) |
| Bovine | M. longissimus thoracis M. longissimus lumborum (LL) M. psoas major M. semimembranosus |
Fresh: Aging at 2°C for 0, 7, and 14 days Freeze-thawing: Frozen at −18°C for 0, 3, 6, and 9 months |
↓ CIE L* in PM with increase of freezing period - CIE a* and CIE b* by aging and freezing period ↑ WHC in LL, PM, and SM by freezing for 3 months and in LT by freezing for 3 and 6 months ↑ Cooking loss in LL, LT, PM, and SM with an increase of freezing period ↓ WBSF in LL, LT, and SM with aging period |
Cho et al. (2017) |
| Porcine | M. longissimus dorsi | Freeze-thawing: –20°C, –30°C, and –40°C in airflow velocities of 0, 1.5, and 3 m/s and thawing at 2°C over night Ice crystal size Category I: Slow freezing in <0.4 cm/h Category II: Intermediate freezing in 0.6–0.7 cm/h Category III: Rapid in >0.96 cm/h |
Ice crystal size: Slow freezing (<0.5 cm/h)>rapid freezing (>0.5 cm/h) ↓ Thawing loss in category III>II>I ↓ Drip loss and cooking loss in category III>II>I ↓ CIE a* and CIE b* in category I ↓ WBSF in category II and III than at –20°C with no air |
Yun et al. (2021) |
| Porcine | M. longissimus dorsi | ISF: Fast freezing in freezing solution at −22°C consisting of 16% sodium chloride, 25% ethyl alcohol, 1.2% chitosan, 0.8% antifreeze protein, and 57% water and thawing at 1, 31, 61 and 91 days at 4°C for 12 h AF: Slow freezing in air-blast at −22°C and thawing at 1, 31, 61 and 91 days at 4°C for 12 h |
↓ Ice crystal formation in ISF ↓ WBSF in AF ↑ Myofibrillar damage and lipid oxidation in AF - CIE L*, CIE a*, and CIE b* in ISF and AF with thawing for 91 days ↑ pH in AF at 91 days, - pH in ISF ↑ Thawing loss in AF for 1, 31, and 91 days ≒ Cooking loss in AF and ISF |
Hou et al. (2020) |
| Bovine | M. longissimus thoracis | Fresh: Aged at 2°C for 2, 3, 4, 5, 6, 7, 10, 14, 21, and 35 days Freeze-thawing: Aged for 3, 4, 5, 6, 7, 10, 14, 21, and 35 days at 2°C, frozen at −16°C for 2 months and thawed at 1°C for 24 h |
Cooking loss: Fresh<frozen-thawed for 1, 3, 4, 5, 6, 7, and 10 days WBSF: Fresh>frozen-thawed for all days ≒ WBSF for 3, 6, and 7 days aged-frozen meat with aged for 14 to 21 days fresh meat |
Shanks et al. (2002) |
| Bovine | M. longissimus thoracis et lumborum | Pelvic suspension: Carcasses hung in pelvic bone Achilles suspension: Carcasses hung in Achilles tendon Both suspensions treated at 4°C, aged for 2, 4, 7, 14, and 21 days and frozen at −20°C for 7 days, and thawed at 4°C overnight |
- Tenderness in both suspensions ↓ WBSF in pelvic suspension for 2 days ageing ≒ WBSF between suspensions after ageing for 14 and 21 days ≒ pH in both groups during ageing ↓ WHC in Achilles suspension |
Enfält et al. (2004) |
| Bovine | M. longissimus thoracis | Fresh: Aged at 4°C for 3 and 10 days Freeze-thawing: Aged at 4°C for 3 and 10 days and frozen at −20°C and −80°C for 30, 75, and 90 days, and thawed at 4°C for 48 h |
↓ CIE L*, CIE a*, and chroma in fresh aged for 3 and 10 days ↓ CIE a*, CIE b*, and chroma, and ↑ hue in freeze-thawing for 90 days ↑ Freezing loss and press loss in fresh aged for 3 and 10 days - Cooking loss in freeze-thawing for storage periods ↑ Press loss in freeze-thawing for 90 days ↑ Cooking loss in freeze-thawing for 30, 60 and 90 days ↓ WBSF in fresh aged for 3 days and freeze-thawing for 75 and 90 days - WBSF in Fresh aged for 10 days ↑ Tenderness in freeze-thawing aged for 3 days and frozen for 30, 75 and 90 days |
Vieira et al. (2009) |
| Porcine | M. psoas major | Freezing Fresh: Stored in 4°C for further analysis SR: −10°C supercooled rapid freezing in static air freezer at −80°C NR: Non-supercooled rapid freezing at −80°C SS: −10°C supercooled slow freezing at −20°C NS: Non-supercooled slow freezing at −20°C Thawing RT: Room temperature thawing in 20°C IW: Ice water thawing at 0±0.5°C RF: Refrigerator thawing at 4°C TW: Tap water thawing under 12±2°C running water |
- Hardness in all freeze-thawing condition Drip loss: IW and RF<TW and RT ↑ Drip loss in NS ↓ Size ice crystal in SR |
Poudyal et al. (2023) |
| Bovine | M. psoas major | Freezing Fresh: Stored at 4°C for 2 days Freezing: Frozen at −40°C for overnight Thawing POT: Pressure ohmic thawing up to 200 MPa and electric field strength of 40 V/cm PAT: Pressure-assisted thawing up to 200 MPa Ohmic thawing: Ohmically thawed to 8°C under electric field of 40 V/cm to 4°C at 0.1 MPa Conventional thawing: Thawed to 4°C by immersion thawing |
↓ Drip loss in PAT, POT and ohmic thawing Cooking loss: POT<PAT<ohmic thawing<conventional thawing ↑ WBSF in PAT - CIE L* in all condition ↓ CIE a* and CIE b* in PAT, ohmic thawing and conventional thawing |
Min et al. (2016) |
| Bovine Porcine Lamb |
M. psoas major | Freezing Fresh: Refrigeration temperature Freezing: At −80°C for 2 days Thawing MT: Microwave thawing UT: Ultrasonic thawing IT: Infrared thawing at 60°C and the power of the infrared tube to 12 W RTT: Thawed at room temperature SWT: Thawed in still water at 25°C |
↓ Thawing loss and cooking loss in UT ↑ Cooking loss in RTT ↑ CIE L* in RRT ↓ CIE L* in UT ↑ CIE b* in UT ↓ Lipid oxidation in UT |
Gan et al. (2022) |
Regardless of the animal species, increasing the freezing rate can reduce the size of ice crystals and the thawing loss (Kim et al., 2020; Yun et al., 2021). Repeated freeze-thaw cycles result in a significant deterioration of meat quality; additionally, a substantial alteration in freezing temperature results in unfavorable WHC compared to a minor temperature alteration (Wang et al., 2020). The WHC varies based on the order of aging (cold storage) and freezing. When aged for approximately three weeks following freeze-thawing, more exudates were lost compared to frozen-thawed following aging (Kim et al., 2018). In studies using various cuts (muscles) of pork and beef, the loss of exudates owing to freeze-thaw may vary based on the muscle type. Cooking loss of pork loin (M. longissimus thoracis et lumborum) increased significantly following freeze thawing compared to that of other muscles (M. psoas major, M. semimembranosus, and M. semitendinosus). However, in beef, tenderloin (M. psoas major) exhibited significant WHC following freeze thawing compared to other muscles (M. longissimus lumborum, M. semimembranosus, and M. semitendinosus; Cheng et al., 2020; Cheng et al., 2021). Additionally, it has been confirmed that cooking loss in beef significantly increases when the freezing period increases, regardless of muscle type (Cho et al., 2017). Freezing solutions consisting of sodium chloride, ethyl alcohol, and chitosan reduce the size of ice crystals and the thawing-related loss in pork (Hou et al., 2020).
During thawing, meat undergoes protein breakdown, lipid oxidation, color changes, and ice crystal melting, reducing its WHC (Gan et al., 2022; Min et al., 2016). Poudyal et al. (2023) observed that shorter thawing times increased drip loss in porcine M. semimembranosus, whereas longer thawing times enhanced the reabsorption of free water from ice crystals, thereby reducing the final moisture loss. However, shorter thawing times may result in inadequate water reabsorption, resulting in excessive water loss (Gonzalez-Sanguinetti et al., 1985). Min et al. (2016) observed that thawing meat using pressure ohmic thawing of up to 200 MPa with an electric field strength of 40 V/cm and pressure-assisted thawing of up to 200 MPa reduced drip loss in beef psoas major compared to conventional thawing methods.
Consumers prioritize price and color when purchasing meat (Carpenter et al., 2001). However, meat discoloration occurs during storage or retail displays. Based on previous research, 15%–20% of discoloration occurs during retail displays, resulting in an economic loss of 50% or more (Hood and Riordan, 1973; Mancini and Hunt, 2005). The primary factors influencing meat color are the myoglobin content and chemical state (Suman and Joseph, 2013). Myoglobin alters meat color based on redox phenomena (King et al., 2023). In its unoxidized state containing iron molecules, myoglobin exists as deoxy-myoglobin (deoxyMb), and the meat color appears purple. When deoxyMb reacts with oxygen in the air, known as blooming, it transforms into oxy-myoglobin (oxyMb), which displays a bright cherry-red color. When deoxyMb and oxyMb lose electrons or both electrons and oxygen, they are converted into oxidized myoglobin (metmyoglobin; metMb), resulting in meat with a brownish tint.
Therefore, meat color changes based on the chemical state (oxidation or reduction states, bonding with oxygen or water molecules) of the heme iron molecule in myoglobin. Additionally, the role of oxidation-reduction enzymes in altering the chemical state is crucial (Tang et al., 2005a; Tang et al., 2005b; Tang et al., 2005c). External factors that affect the oxidation-reduction states of myoglobin, such as packaging conditions (aerobic vs. anaerobic), storage temperature (refrigerated vs. frozen), and storage period, directly alter the oxidation, reduction, and oxygenation states (King et al., 2023). Moreover, it also affects the activity of enzymes involved in the oxidation-reduction of myoglobin (Tang et al., 2005a; Tang et al., 2005b; Tang et al., 2005c). Freeze thawing is one of the factors responsible for these intricate alterations in meat color (Jeong et al., 2011).
As demonstrated in Table 1, a slow freezing speed correlated with an increase in the CIE L* of the meat. Moreover, with an extended frozen storage period, the CIE L* of beef tenderloin reduces (Cho et al., 2017; Hou et al., 2020; Kim et al., 2020). However, the CIE a* and CIE b* of pork and beef do not vary during the freezing period (Cho et al., 2017; Hou et al., 2020). Additionally, repeated freeze-thaw with significant temperature alteration results in a reduction in the CIE L* and CIE a* of beef, whereas the CIE b* increases (Wang et al., 2020).
Meanwhile, during cold storage following freeze-thaw, a slower freezing speed results in an increase in the CIE L* of pork loin and a reduction in CIE b* (Kim et al., 2018). Studies on the effects of freeze-thaw on pork and beef muscles have shown contrasting results for the loin muscles of the two species. The CIE L* of beef was reduced and the CIE a* increased following freeze-thawing, whereas CIE a* increased following freeze-thawing (Cheng et al., 2020; Cheng et al., 2021). However, the CIE L* of pork loin tends to be increased and CIE a* is reduced following freeze-thawing (Cheng et al., 2021). In contrast, semimembranosus exhibited an increase in CIE a* owing to freeze-thawing, regardless of the species. Moreover, psoas major exhibited a tendency to reduce CIE b* owing to freeze-thawing of beef. However, psoas major exhibited relatively smaller alterations in meat color owing to freeze-thawing in both species compared to other muscles, such as M. longissimus thoracis et lumborum, M. semimembranosus, and M. semitendinosus (Cheng et al., 2020; Cheng et al., 2021). Furthermore, the meat color changes during thawing. Min et al. (2016) demonstrated that thawing beef psoas major did not alter the CIE L* of meat. However, the CIE a* and CIE b* reduced in all thawing treatments, such as pressure-assisted thawing up to 200 MPa, pressure ohmic thawing up to 200 MPa, electric field strength of 40 V/cm, and immersion thawing. Gan et al. (2022) observed that ultrasonic thawing for beef, pork, and lamb M. psoas major resulted in minimal alterations in meat color compared to other thawing methods, such as microwave, room temperature, and 25°C water thawing. This indicates that ultrasonic thawing is significantly effective in preserving muscle quality and reducing myoglobin and lipid oxidation in meat.
Meat tenderness is a crucial sensory quality, and its variations are caused by alterations in the chemical composition and structural properties of muscle fibers and connective tissues, which are influenced by the animal species, breed, slaughter method, and postmortem processing of the meat (Pogorzelski et al., 2022; Zhang et al., 2023). Freezing affects meat tenderness with a positive correlation between freezing and tenderness (Lagerstedt et al., 2008). Ice crystals generated through freezing exert pressure on muscle tissues, resulting in physical destruction and protein decomposition (Añón and Calvelo, 1980; Cheng et al., 2020). When meat is frozen, larger ice crystals cause significant physical destruction of the muscle tissue and meat tenderness (Leygonie et al., 2012; Zhang et al., 2023). Thawing technique also affects meat tenderness. Min et al. (2016) demonstrated that the pressure ohmic thawing technique results in minimal texture alteration, with shear forces closely resembling those of fresh meat.
Aging the meat adequately before freezing negates the tenderizing effect of freezing (Vieira et al., 2009). Enfält et al. (2004) discovered that beef subjected to initial aging for seven days before freezing exhibited similar shear force to beef aged for 21 days in refrigeration. Shanks et al. (2002) observed that frozen-thawed longissimus dorsi exhibited a lower shear force than chilled meat over the same period. Freezing enhances tenderness (reduction in shear force); however, the degree of enhancement varies based on the freezing rate. Rapid freezing forms smaller ice crystals compared to slow freezing, resulting in relatively less enhancement of tenderness (Kim et al., 2015; Kim et al., 2018; Yun et al., 2021). Additionally, prolonged frozen storage enhances meat tenderness (Cho et al., 2017). Furthermore, distinct tenderness patterns following freeze-thawing were observed among various muscle types, with a reduction in the tenderness of porcine M. semimembranosus and M. semitendinosus, and an enhancement in the tenderness of bovine M. longissimus lumborum (Cheng et al., 2020; Cheng et al., 2021). Cheng et al. (2020) and Cheng et al. (2021) reported that varied characteristics of the muscle fibers in each muscle may contribute to the varying susceptibilities of muscle types owing to freezing. Additionally, freezing can physically damage muscle tissue and enhance tenderness but weakens the WHC of meat. This leads to excessive meat exudation upon thawing, resulting in a dry or tough meat texture. Because excessive exudation of meat juice negatively affects tenderness, it can be enhanced or reduced through freeze-thawing based on the characteristics of each muscle. In order to reduce the negative changes in meat texture caused by the reduction in WHC, it is necessary to minimize the destruction of the tissue through rapid freezing and high pressure treatments (Choi et al., 2016; Min et al., 2016; Sanz et al., 1999).
Freezing is a microbiologically safer and long-term meat storage method compared to refrigeration. This is because microbial growth is inhibited at approximately –12°C, cell metabolism in animal tissue is inhibited at approximately –18°C, and alterations in meat quality are minimal at –55°C (Dave and Ghaly, 2011; Hansen et al., 2004). Beef and lamb can be stored at –18°C for 12 months, pork for six months, and poultry meat for 9–12 months (Valero Díaz et al., 2023). Freezing inhibits microbial growth and kills approximately 60% of the microbial population. However, surviving microbes proliferate upon thawing, and fat oxidation persists even during freezing (Dave and Ghaly, 2011; Zhou et al., 2010). Therefore, freezing does not ensure the safety of meat. Lipid oxidation and meat spoilage bacteria are significant factors that shorten the shelf life of meat. Meat products undergo oxidation during processing, storage, and light exposure. This process deteriorates the nutritional and organoleptic properties of meat and results in the formation of toxic compounds that are harmful to humans (Papuc et al., 2017).
Thawing results in the conversion of solid ice crystals to liquid water, which raises the surface temperature and can facilitate microbial reactivation by providing appropriate temperature and humidity conditions (Marriott et al., 1980). Additionally, juices exuded during freezing and thawing provide a nutritious environment and suitable medium for microbes (Leygonie et al., 2012). Rapid thawing techniques are crucial for minimizing microbial growth (Min et al., 2016) and reducing damage caused by thawing.
Bacteria significantly associated with meat spoilage include Carnobacterium spp., Enterobacteriaceae, Lactobacillus spp., Leuconostoc spp., Shewanella putrefaciens, Pseudomonas spp., and Brochothrix thermosphacta (Borch et al., 1996). Pseudomonas species are the primary cause of meat spoilage at temperatures between –1°C and 25°C in air. Pseudomonas can penetrate meat because of its proteolytic activity (Gill and Penney, 1977; Gupta and Nagamohini, 1992). Therefore, bacterial spoilage of meat results in odors, off-flavors, discoloration, gas production, slime formation, and pH reduction, thereby causing significant economic losses (Papuc et al., 2017).
The primary factor that causes the deterioration of stored meat is lipid oxidation, which is caused by enzymes produced by microbes and meat enzymes or autoxidation (Zhang et al., 2010). Among microbes, certain isolates produce lipid-oxidizing enzymes, such as lipoxygenase, linoleate, oxygen oxidoreductase, and hydroperoxide lyase (Brash, 1999). Meat oxidation is a significant factor in the deterioration of meat quality, affecting flavor, texture, nutritional value, and color. Lipid oxidation in fresh meat, known as autoxidation, occurs during cold or frozen storage and is induced by oxygen and lipid free radicals (Soyer et al., 2010). Free radicals are atoms or molecules characterized by unpaired electrons that are inherently unstable and reactive in nature. When they attract electrons from stable compounds, they become free radicals, creating a continuous cycle (Fang et al., 2002; Škrovánková et al., 2012). The resulting hydroperoxides cause biological damage to lipids, hexanes, enzymes, and proteins, thereby resulting in the production of detrimental compounds, such as malondialdehyde and cholesterol oxidation products (Morrissey et al., 1998). Numerous factors influence lipid oxidation in meat, including heat, light, antioxidants, oxygen, phospholipid, and unsaturated fatty acid contents (Guyon et al., 2016). Gan et al. (2022) observed that freeze-thawing of psoas major from bovine, porcine, and lamb increased lipid oxidation in meat across all animal species. However, ultrasonic thawing resulted in significant stable alterations. The degree of lipid oxidation increases with longer thawing times (Fioramonti et al., 2017; Gan et al., 2022). Microwave thawing has a shorter thawing time; however, because of the energy and heat generated, it is excessive and facilitates lipid oxidation (Gan et al., 2022; Lorentzen et al., 2020). Phospholipids present in cell membranes are highly sensitive to oxidation because of their higher unsaturation compared with other fats in meat (Domínguez et al., 2019). Lean meat contains a relatively high proportion of easily oxidized phospholipids. Therefore, phospholipids are the primary cause of fat oxidation in lean meats (Domínguez et al., 2019). Additionally, phospholipid fat oxidation results in noticeable alterations in the meat quality. Lipase-mediated phospholipid degradation has also been observed in frozen meat. These reactions occur at various freezing temperatures and storage periods (Pikul et al., 1985). Therefore, the degree to which packaging reduces air exposure during frozen storage plays a crucial role in determining fatty acid oxidation. Protein oxidation, assessed through carbonyl and sulfhydryl content, is significantly correlated with lipid oxidation (Mercier et al., 1998; Renerre et al., 1999). Meat, specifically chicken, has a high unsaturated fatty acid content. Therefore, lipid and protein oxidation are more prevalent during frozen storage. Moreover, the degree of oxidation is highly significant in chicken legs than in chicken breasts (Soyer et al., 2010).
Assessing Individual Muscle Characteristics to Enhance Meat Freezing/Thawing Technologies
The quality of meat subjected to freezing and thawing depends on pretreatment, freezing, storage, and thawing conditions. Freezing is the primary process that significantly influences the overall quality of frozen muscle food (Nakazawa and Okazaki, 2020). Rapid freezing and the formation of small ice crystals are crucial for meat freezing because they reduce muscle tissue damage and drip loss during thawing (Li and Sun, 2002). Kim et al. (2018) demonstrated that total exudate loss was reduced in pork loin muscle subjected to rapid freezing (–80°C) compared to slow freezing (–20°C). Moreover, aging before freezing and rapid freezing contributed to reduced deterioration. Additionally, rapid freezing reduces the purge and drip loss in beef loins (Kim et al., 2015). Kim et al. (2020) discovered that preserving pork neck and chicken leg meat at varying storage temperatures (–18°C, –50°C, and –60°C) for six months revealed that maintaining the quality of fresh meat without any degradation for the complete duration was achieved through freezing at –60°C. High-pressure freezing is anticipated to achieve significant supercooling effects that can form uniform ice crystals within meat products (Cheftel et al., 2002). Upon pressure release, an increase in pressure results in the transformation of type-I ice crystals (less dense than liquid water) to type-IV ice crystals (Cheftel et al., 2000). Type IV ice crystals, which are smaller and denser than water, exhibit no expansion upon freezing, in contrast with the 9%–13% typical expansion observed in type I crystals. They can produce high-quality freeze-thawed meat with minimal cell structure damage. However, high-pressure freezing is expensive and has limitations on meat size (Chevalier et al., 2000; Fernández et al., 2007). Additionally, static electric field freezing (Fallah-Joshaqani et al., 2019) and ultrasonic technology (Patist and Bates, 2008) have been proposed, with ultrasonic technology reducing the thawing time and structural damage to muscle fibers owing to freezing (Guo et al., 2021).
The degree of alteration in meat quality owing to freezing and freeze-thaw varies based on the moisture and fat content and muscle fiber characteristics (Huff-Lonergan and Lonergan, 2005; Song et al., 2020). Among the muscle fiber types, type I is less susceptible to freezing than type II. Therefore, muscles with a higher type I distribution exhibit less deterioration in meat quality when subjected to freezing and thawing (Cheng et al., 2020; Cheng et al., 2021; Song et al., 2020). Moreover, various cuts/muscles exhibit distinct muscle fiber characteristics, including muscle fiber composition, resulting in distinct freezing susceptibilities. Variations in freezing susceptibility based on muscle fiber type have been confirmed in major cuts (loin or strip loin, tenderloin, round, and eye of round) of beef and pork (Cheng et al., 2020; Cheng et al., 2021). Beef tenderloin (M. psoas major) with a high proportion of type I muscle fibers exhibited a relatively lower degree of reduction in WHC and discoloration owing to freeze-thawing compared to round (M. semimembranosus) and eye of round (M. semitendinosus) with a high proportion of type II muscle fibers. This pattern was also observed in the WHC and tenderness of pork. Therefore, these results emphasize the significance of freeze-thaw that considers the components or muscle fiber characteristics of each muscle/cut, regardless of the animal species.
Thawing meat results in ice crystal melting, thereby reducing WHC. Therefore, it is crucial to use appropriate thawing techniques to enhance the quality of thawed meat (Alonso et al., 2016), prevent damage to the muscle structure, minimize fat and protein oxidation, and reduce water retention (Zhang et al., 2023). Generally, meat is thawed using air or water at refrigeration or room temperature, which provides a cost-effective approach without physical treatment (Gan et al., 2022; Lan et al., 2021). Advanced thawing technologies, including high-pressure, microwave, ohmic, ultrasonic, electrostatic field, and radiofrequency field and combinations of these techniques (Zhang et al., 2023), can effectively reduce the thawing time and minimize the deterioration of meat quality compared to traditional thawing techniques. Gan et al. (2022) reported that ultrasonic thawing, a physical technique, can effectively prevent muscle structure deformation in meat. Although shorter thawing times are known to reduce meat deterioration, Lan et al. (2021) demonstrated that microwave thawing results in serious muscle damage owing to regional heating. However, a study discovered that radiofrequency thawing can reduce alterations in the structure and properties of proteins, thereby increasing the thawing efficiency.
Each skeletal muscle has its own morphological, physiological, kinematic, and functional characteristics. However, muscle cells (fibers) exhibit varying physicochemical characteristics (Park et al., 2022). As demonstrated in Table 2, representative porcine skeletal muscles were identified based on their varying compositions of muscle fiber types (I, IIA, IIX, and IIB), resulting in varied pH, meat color, WHC, and tenderness (Chang et al., 2003; Park et al., 2022; Ruusunen and Puolanne, 2004). Moreover, various bovine skeletal muscles have been profiled and their fiber characteristics and physicochemical properties were differentiated (Joo et al., 2017; Lang et al., 2020; Von Seggern et al., 2005). As reported by Cheng et al. (2020) and Cheng et al. (2021), various levels of quality alterations through freeze-thaw process are anticipated between various muscle types exhibiting varying muscle fiber characteristics and meat quality. Specifically, porcine skeletal muscles with a higher type II fiber composition and lower type I fiber composition, such as M. longissimus dorsi, M. gluteus superficialis, M. semimembranosus, and M. semitendinosus should avoid freezing because type II fibers are highly susceptible to freezing than type I fibers (Chang et al., 2003; Park et al., 2022; Ruusunen and Puolanne, 2004; Song et al., 2020). For bovine skeletal muscles, M. psoas major and M. superficialis flexors are anticipated to remain stable when frozen owing to their higher type I fiber composition than that of type II fibers. In contrast, bovine M. semitendinosus, M. semimembranosus, and M. gluteus medius, which consist predominantly of type IIB fibers, are anticipated to be susceptible to freezing (Joo et al., 2017; Lang et al., 2020). The muscles are prone to instability during freezing, specifically in pork loin and round cuts, in contrast to the highly stable muscles during freezing, such as tenderloin and a part of the shank. Until recently, with only limited studies, alterations in meat quality owing to freezing and thawing, considering the muscle fiber composition of each cut, have not been adequately assessed. Furthermore, no freezing and thawing technologies were introduced.
| Species | Breed | Sex/age/carcass weight | Muscles | Outcome | References |
|---|---|---|---|---|---|
| Porcine | Berkshire Tamworth Duroc Large White |
Male/NP/NP | M. longissimus dorsi M. psoas major |
↑ Type I, muscle fiber proportion in M. psoas major of the Berkshire ↑ Type I, muscle fiber proportion in M. longissimus dorsi of the Large White ↑ Type IIA, muscle fiber proportion in M. psoas major than in M. longissimus dorsi ↑ Type IIB and ↓ type IIA, muscle fiber amount in M. longissimus dorsi of Berkshire, Duroc and Tamworth ↓ Type I, muscle fiber amount in Large White and ↑ type I amount in both muscles of Duroc ↑ Type IIA, muscle fiber amount in Duroc and ↓ type IIA amount in Large White and Tamworth ↓ Type IIb and ↓ type IIX, muscle fiber amount in M. psoas major of Large White ↓ pH at 45 min, drip loss, CIE L*, and hue value in M. psoas major than M. longissimus dorsi ↑ pH at 24 h, CIE a* and CIE b* in M. psoas major than M. longissimus dorsi |
Chang et al. (2003) |
| Porcine | Wild Domestic |
Male and female/165±2 days/105.1±8.8 kg | M. longissimus dorsi M. gluteus superficialis M. semimembranosus M. infra spinam M. masseter |
↓ Type IIB and ↟type IIA, muscle fiber area % in M. longissimus dorsi, M. gluteus superficialis, and M. semimembranosus of wild pig ↑ Type IIB, muscle fiber area % in M. longissimus dorsi, M. gluteus superficialis, and M. semimembranosus in domestic pig ↑ Type IIA and ↓ type IIB, muscle fiber area % in M. infra spinam of wild pig ↑ Type I, muscle fiber area % in M. masseter of wild pig |
Ruusunen and Puolanne (2004) |
| Porcine | LYD | Castrated/NP/79.2±3.5 kg | M. infrahyoid M. rectus abdominis M. gracilis M. psoas major M. semitendinosus M. semimembranosus M. vastus M. diaphragm M. longissimus dorsi M. biceps brachii M. biceps femoris M. rectus femoris M. subscapularis M. superficialis digital flexor |
↓ Moisture content, and high CIE L*, cooking loss, and WBSF in M. semitendinosus than in M. psoas major and M. vastus ↑ Moisture, crude protein, and WBSF but ↓ fat content, pH, CIE a*, and CIE b* in M. longissimus dorsi than in M. diaphragm ↓ Moisture content, higher crude fat content, and ↑ CIE b* in M. biceps femoris than in M. biceps brachii and M. rectus femoris ↑ pH and WBSF, and ↓ drip loss in M. subscapularis than in M. superficialis digital flexor ↑ All muscle fiber area in M. psoas major than in M. semimembranosus, M. vastus, and M. semitendinosus ↓ Type I, relative fiber area in M. semitendinosus ↓ Type IIA and IIXB, relative fiber area in M. semimembranosus ↑ Type IIA, relative fiber area in M. diaphragm than in M. longissimus dorsi ↑ Type IIX and ↓ type IIB in M. biceps brachii than in M. biceps femoris and M. rectus femoris ↓ Type IIA, muscle fiber density in M. biceps femoris ↑ Type IIX and ↓ type IIB in M. biceps brachii than in M. biceps femoris and M. rectus femoris ↓ Type IIA, muscle fiber density in M. biceps femoris |
Park et al. (2022) |
| Bovine | Chinese Simmental cattle | Bull/26 months/378±30 kg | M. longissimus thoracis M. psoas major M. semitendinosus |
↑ Type I, IIA, and IIB muscle fiber density in M. longissimus thoracis and M. semitendinosus than in M. psoas major ↑ Type I, muscle fiber number % in M. psoas major. ↓ Type I, muscle fiber number % in M. semitendinosus ↑ Type IIB, muscle fiber number % in M. semitendinosus. ↓ Type IIB, muscle fiber number % in M. psoas major ↑ WBSF in M. semitendinosus and M. longissimus thoracis than in M. psoas major ↓ WBSF, hardness, and chewiness values in M. psoas major than in M. semitendinosus and M. longissimus thoracis ↓ Pressing loss in M. semitendinosus and M. longissimus thoracis at 1, 3, and 21 days of aging ↑ Cooking loss in all muscles at 1 and 3 days of aging ↑ CIE L* in M. semitendinosus at 1 and 21 days of aging ↑ CIE a* in M. psoas major at 1 and 3 days of aging ↑ CIE b* in M. semitendinosus than in M. psoas major at 7, 14, and 21 days of aging |
Lang et al. (2020) |
| Bovine | Hanwoo | Steer/NP/NP | M. longissimus lumborum M. psoas major M. semimembranosus M. semitendinosus M. gluteus medius M. triceps brachii M. rectus abdominis M. superficialis flexor |
↑ Type I, muscle fiber number % in M. superficialis flexor ↑ Type IIA, muscle fiber number % in M. psoas major and M. triceps brachii ↓ Type IIA, muscle fiber number % in M. rectus abdominis Type IIB fiber area %: M. semitendinosus>M. semimembranosus>M. longissimus lumborum ↓ Type IIB, muscle fiber number % in M. superficialis flexor ↑ Type I, muscle fiber area % in M. superficialis flexor and ↓ Type I, muscle fiber area % in M. semitendinosus ↑ Type IIA, muscle fiber area % in M. gluteus medius and ↓ Type IIA, muscle fiber area % in M. longissimus lumborum ↑ Moisture and collagen contents in M. superficialis flexor and ↓ moisture and collagen contents in M. longissimus lumborum ↑ CIE a* in M. psoas major and M. superficialis flexor and ↓ CIE a* in M. semimembranosus ↑ CIE L* in M. longissimus lumborum and ↓ CIE L* in M. superficialis flexor ↑ Drip loss and cooking loss in M. semimembranosus ↓ Drip loss and cooking loss in M. longissimus lumborum and M. psoas major ↑ WBSF in M. superficialis flexor and ↓ WBSF in M. psoas major |
Joo et al. (2017) |
| Bovine | NP | NP/NP/250–431 kg | Chuck 27 muscles Round 12 muscles |
↑ Fat content in M. cutaneous omo brachialis, M. longissimus costarum, M. multifidus and spinalis dorsi, M. serratus ventralis, and M. superficial pectoral ↑ WHC in M. deltoideus and M. supraspinatus ↑ WBSF in M. biceps brachii, M. infraspinatus, M. levatores costarum, M. multifidus and spinalis dorsi, M. serratus ventralis, M. subscapularis, and M. teres major ↑ pH in M. dorsalis oblique, M. longissimus costarum, M. levatores costarum, M. multifidus and spinalis dorsi, M. rhomboidus, M. serratus ventralis, M. subscapularis, M. supraspinatus, and M. trapezius |
Von Seggern et al. (2005) |
However, the effects of meat components, such as moisture, fat, protein, and collagen on alterations in muscle tissue and quality characteristics during freezing and thawing have not been assessed. In pork and beef, various muscles exhibit distinct characteristics, resulting in diverse proximate compositions and meat quality properties (pH, color, tenderness, and WHC; Table 2). For example, the porcine M. psoas major has a higher pH than M. longissimus dorsi, whereas biceps femoris has a lower moisture content and higher fat content compared to M. biceps brachii and M. rectus femoris (Park et al., 2022; Ruusunen and Puolanne, 2004). Additionally, M. superficialis flexor exhibits higher moisture and collagen content, whereas M. longissimus lumborum exhibits lower moisture and collagen content compared to the major bovine muscles (Table 2; Joo et al., 2017). Among the various muscles of beef chuck and round cuts, a higher fat content was observed in M. cutaneous omo brachialis, M. longissimus costarum, M. multifidus, M. spinalis dorsi, M. serratus ventralis, and M. superficial pectoral (Table 2; Von Seggern et al., 2005). Moreover, pH, moisture, fat, collagen, WHC, and shear force are anticipated to influence the freeze-thawed meat quality characteristics.
Conclusion
Methods such as rapid freezing, high-pressure treatment freezing and thawing, electric field, ultrasonic treatment thawing, and adjusting the sequence of aging and freezing reduce the degradation of the quality of meat subjected to freezing or freeze-thawing. Regardless of the type of species and cuts (muscle type), improved quality can be expected in the frozen-thawed meat by applying these technologies. Additionally, susceptibility to freezing and quality alterations in meat vary based on the characteristics of each muscle (meat cut). Proper freezing or thawing treatment considering the unique characteristics of each muscle (specifically the muscle fiber characteristics) is expected to further reduce the deterioration in meat caused by freezing. Therefore, additional research is required to assess the effects of unique muscle characteristics (proximate composition, pH, WHC, tenderness, muscle fiber characteristics, etc.) on alterations in the quality of frozen-thawed meat. In conclusion, freezing is a hygienic and safe method to extend the shelf life of meat. However, it is essential to consider unique meat characteristics when implementing technical enhancements to minimize the adverse effects of freeze-thawing on meat quality.













