Introduction
There is a growing trend toward the increased use of probiotics in industrial aquaculture due to growing consumer demand for natural foods, where harsh chemicals and antibiotics are replaced by natural growth promoters and health modulators (Cruz et al., 2012; El-Saadony et al., 2021; Hoseinifar et al., 2018; Irianto and Austin, 2002; Shefat, 2018). Many researchers have reported potential probiotic strains of microorganisms isolated from aquaculture, both from the environment and farmed species. These include lactic acid bacteria such as Lacticaseibaciullus casei (formerly Lactobacillus casei), as well as spore-forming bacilli, including Bacillus subtilis, Bacillus amyloliquefaciens, and Bacillus sonorensis. Spore-forming Bacillus probiotics include strains of Bacillus sp. S11, Bacillus sp. 48, representatives of Bacillus megaterium, Bacillus polymyxa, and Bacillus licheniformis (Merrifield et al., 2010). Many of these probiotic cultures produce amylases, lipases, proteases, phosphatases, cellulases, and other enzymes that have a beneficial effect on the activity of the gastrointestinal tract (GIT) of fish (Merrifield et al., 2010).
A series of studies have shown the positive effect of probiotics related to Lacticaseibacillus rhamnosus (formerly Lactobacillus rhamnosus) and L. casei on the reproductive activity of fish (Aydin and Çek-yalniz, 2019; Gioacchini et al., 2012; Qin et al., 2014; Rohani et al., 2022). Aquabiotics (probiotics for aquaculture) based on strains of Vibrio alginolyticus are widely used in farmed aquacultures (Martínez Cruz et al., 2012), although many representatives of this species are identified as fish pathogens. A probiotic based on V. alginolyticus can reduce fish mortality from infectious diseases, can successfully replace antibiotics in cases of infection prevention, and can also protect salmon from consecutive infection with Aeromonas salmonicida and Vibrio anguillarum (Austin et al., 2010). Transfer of aquabiotics to their intended aquaculture targets occurs in two ways: as a feed additive or directly into the water (Martínez Cruz et al., 2012).
One of the challenges of intensive fish farming is the creation of new biotechnologies for rearing fish using compound feed containing modern probiotic preparations (Ageyets et al., 2019; Ige, 2013). For the development of effective technology for rearing fish, work was carried out to study the effectiveness of the use of feed additives ‘ProStor’, ‘Ferm KM-1’, and ‘Gerbastor’ (Pavlov et al., 2014; Pavlov et al., 2015; Ushakova et al., 2021; Zuenko et al., 2017). An analysis of fish breeding and biological indicators when feeding fish compound feeds containing probiotic preparations showed that the largest weight gain was observed in fish that consumed the feed with probiotics.
Spore-forming Bacillus aquabiotics have attracted the attention of researchers, especially in the last decade. A phytogenic aquabiotic, Bacillus siamensis B44v, increased fish growth when used as a feed additive (107 CFU/g feed) and also doubled fish survival against infection (intraperitoneal injection) with Aeromonas hydrophila (Meidong et al., 2017). A multimicrobial preparation consisting of B. amyloliquefaciens 54A and Bacillus pumilus 47B improved fish growth and prevented Edwardsiella ictaluri infection, significantly reducing mortality (Thy et al., 2017). Finally, a review articles published in 2020 (Kuebutornye et al., 2020; Olmos et al., 2020; Ringø et al., 2020) emphasized the role of spore-forming bacilli in the health of aquaculture systems and the possibility of their use as aquabiotics.
The recently identified probiotic strains of B. subtilis KATMIRA1933 and B. amyloliquefaciens B-1895 inhibit the formation of pathogenic microorganisms’ biofilms and modulate poultry growth and health. The strain B. subtilis KATMIRA1933 was isolated from dairy products and the strain B. amyloliquefaciens B-1895 was isolated from soil. Our previous studies have shown that these strains have a positive effect on poultry, namely: they increase weight gain, egg production, egg quality, and also reduce the degree of damage to mitochondrial DNA (Chistyakov et al., 2015; Makarenko et al., 2019; Mazanko et al., 2018; Mazanko et al., 2019; Prazdnova et al., 2015; Prazdnova et al., 2019; Tazehabadi et al., 2021).
The purpose of the scientific experiment was to study a probiotic supplement based on B. amyloliquefaciens B-1895 on the growth and evolvement of aquaculture objects.
Materials and Methods
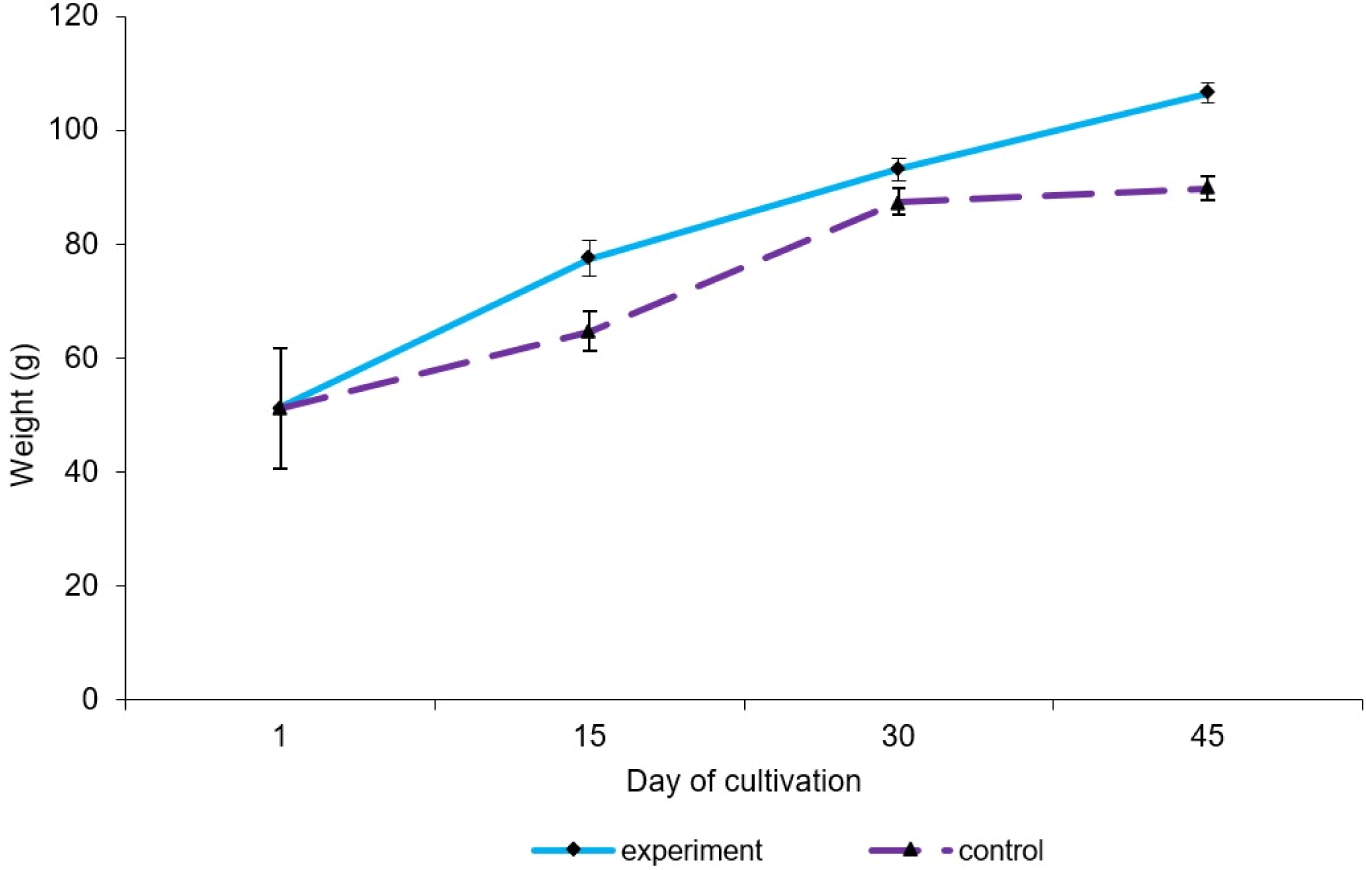
The study was carried out at the Adler Breeding Trout Breeding Plant JSC of the Federal Research Center Southern Scientific Center of the Russian Academy of Sciences, and the Center for Agrobiotechnology, Don State Technical University. The fish used were rainbow trout Oncorhynchus mykiss (Walbaum 1792) underyearlings. The age of the fish was four months. The fish were divided into groups based on body weight indices. The initial weight of underyearlings was similar in both groups (p>0.05); 51.36±10.62 in the experiment group and 51.22±10.50 in the control group.
This report does not contain any studies with human participants or animals that require approval according to the local rules and regulations, as the research is conducted on young fish specially bred for experimental purposes.
Each group of fish was reared separately in round plastic tanks with a volume of 1.2 m3 with a constant flow of 6–8 cm/s. Major hydrochemical parameters were monitored daily. The water temperature was in the range of 13.0°C–15.0°C, and the oxygen content varied from 9.0 to 10.7 mg/L. Each group consisted of 100 fish, and the groups were inspected for external injuries and diseases before the experiment started; only healthy individuals were selected for the experiment. Selection of fish for analysis of biochemical and microbiological parameters was carried out randomly. The number of samples in the sample of 10 in the experiment and 10 in the control is sufficient for this type of research. Fish were fed three times a day (at 9, 15, and 20 h), and the duration of the experiment was 45 days.
A new probiotic preparation was obtained by solid-phase fermentation of soybeans with B. amyloliquefaciens B-1895 as described previously (Chistyakov et al., 2015). The content of bacteria in the preparation was 4×1010 CFU/g as determined by plate counting.
A compound feed with a probiotic additive was used in the experiment while the control feed contained no probiotic. The probiotic additive was mixed into the feed to a final concentration of in the amount of 0.15% (the final bacteria load in the feed was 6×107 CFU/g).
The feed was produced at the enterprise LLC “BIF” (Astrakhan region) according to the state regulation GOST 10385-2014 used for compound feeds for fish (GOST, 2014), with a protein content of 45% and a fat content of 15%. The composition of the feed is as follows: fish meal, meat meal, soybean meal, wheat gluten, fodder yeast, wheat, fish oil, premix, and the probiotic (Table 1).
Only plant-derived and starch-containing components of the feed were subjected to high temperature and pressure processing. This method allows for maximizing the preservation of essential amino acids and vitamins in the animal-derived components. The size of pellets prepared for trout juveniles (10 to 500 g) was 2–3 mm. All feed components were mixed in the SVG-5A mixer. At this stage of production, the probiotic was added in dry form. The resulting blend was passed to a DG-3VU pelleting press (Doza-Agro, Nizhny Novgorod, Russia) for the subsequent formation of granules.
Methods of physiological assessment of the nutritional value of fish feed were used when determining the efficiency of the use of feed by the body. The daily ration was calculated by the formula:
where C: daily feeding rate, kg; Р: the average weight of fish, kg; A: daily ration, % of the mass of fish. The feed coefficient was determined as the ratio of the amount of feed consumed to the weight gain of the fish throughout the experiment.
Analysis of body conditions was conducted to evaluate the living conditions of the studied fish since they are subject to significant fluctuations depending on changes in the biotic and abiotic parameters of the aquatic environment.
Absolute growth was calculated by the formula:
where P: absolute growth, g; Мк: mass at the end of cultivation, g; МО: mass at the beginning of cultivation, g.
The average daily growth was determined by the formula:
The growth rate is the increase in fish weight per unit of time. Rate is an absolute measure of growth over the period in which it is recorded. When using the weight method, it is measured by an increase per day, expressed in grams. The average daily growth rate was calculated using the compound interest formula:
where A: average daily growth rate, %; MK: weight at the end of cultivation, g; MO: weight at the beginning of cultivation, g; t: duration of cultivation, days.
Determination of the mass accumulation coefficient was carried out according to the formula:
where KM: coefficient of mass accumulation, units; MK: weight at the end of cultivation, g; MO: weight at the beginning of cultivation, g; t: duration of cultivation, days.
Survival ability was calculated according to the count of dead trout fry daily.
Sampling and biochemical analysis (moisture, dry matter, protein, fat, and ash) of the fish body were carried out according to conventional procedures (state regulation GOST-7636-85; GOST, 1985). Analysis of the chemical composition of the body of the fish under study was carried out in a specialized laboratory, moisture content – by drying at a temperature of 105°C, fat content – by extraction method in a Soxhlet apparatus, content protein – according to Kjeldahl, ash – by combustion in a muffle furnace at temperature 500°C (Shcherbina, 1983).
The fish was fed three hours before slaughter to ensure normal intestinal contents. Ten fish were selected randomly from each group, and delivered within one day on ice to the laboratory. Upon delivery, the GIT was removed from each fish and the contents of the intestine (both abdominal and parietal) were placed into a sterile container with a scraper. The obtained samples were thoroughly mixed with a sterile glass rod, and a series of successive decimal dilutions were prepared.
Determination of the selected microorganisms in the microbial population of the samples was carried out by the method of surface inoculation in the amount of three replications for each nutrient medium and each dilution studied.
To determine the number of lactic acid bacteria, De Man, Rogosa and Sharpe (MRS) medium (Lenreaktiv, St Petersburg, Russia) was used, Enterococcus medium was used for the isolation of enterococci (HiMedia, Maharashtra, India), Escherichia coli and lactose-positive bacteria (Citrobacter and Enterobacter) were enumerated using Endo medium (HiMedia), and Vibrio representatives were enumerated using selective Vibrio agar (HiMedia). Each sample was spread plated in triplicate. The plates were incubated in a Bactrone anaerobic station (Sheldon Manufacturing, Cornelius, OR, USA) at 37°C. After 48 h of incubation, the resulting colonies were counted.
For isolation and enumeration of Bacillus spores, samples were incubated at 4°C for 48 h, diluted 1:10 with saline, and placed in a water bath for three minutes at 93°C–95°C. As determined by plating, during this time, all microorganisms in the sample died, except for spores of bacilli. Next, a series of consecutive dilutions were made and plated on a solid meat-peptone agar (MPA) nutrient medium (Lenreaktiv) and incubated for 24 h at 37°C, and the resulting colonies were counted.
Data were analysed using SPSS version 20.0 (IBM, Armonk, NY, USA). The primary hypothesis of this study involved differential efficacy between the two treatment groups of probiotic and control. Differences between groups were compared using the Student’s t-test. Comparisons between treatment groups as a measure of time were assessed using between-group repeated measures analysis of variance (ANOVA; general linear model) with group and time as main effects, with additional group-by-time interaction term. All tests were two-sided with p<0.05 as considered statistically significant.
Results and Discussion
Overall, the experimental group that consumed feed with a probiotic supplement based on B. amyloliquefaciens B-1895 had improved growth, survival, and feed conversion compared to the control group (Table 2).
The final weight of the fish was significantly (p≤0.001) higher by 15.7% in the experimental group and reached an average of 106.56±1.85 g. Indicators of absolute and average daily weight gain were higher by 30.1% in fish from the experimental group at 55.20 g and 1.23 g/day, respectively. A higher average daily trout growth rate of 1.62% was noted, which is 0.38% higher than in the control group.
The feed coefficient in the control group was 1.6 units. In the experimental group, the coefficient value is much lower - 1.2 units, which suggests more efficient digestion and assimilation of feed. A higher survival rate for the experimental group was observed compared to the control group at 98% compared to 96%.
The trout gained the most weight when consuming the probiotic-supplemented feed and for the control group the rate of mass accumulation was slower. The mass accumulation coefficient for the control was 0.05 units, while in the experiment, it was 0.07 units (Fig. 1).
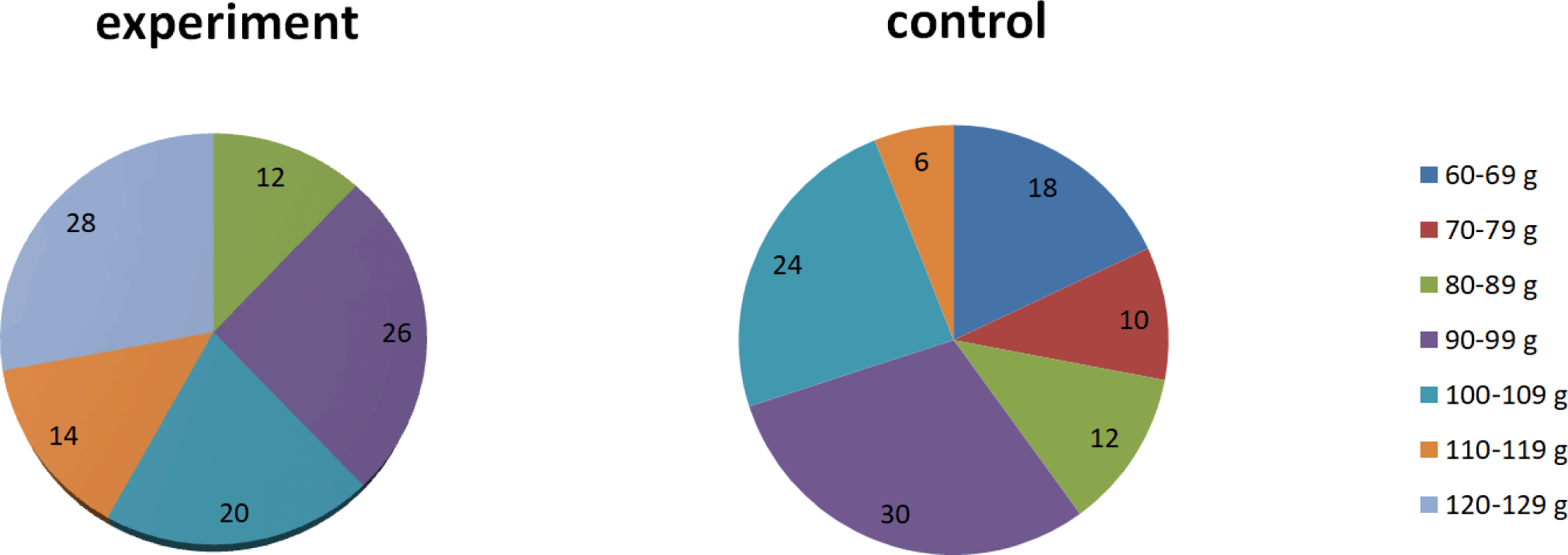
Histogram analysis of the mass structure of trout showed that the juveniles of the control group weighed 60 to 115 g, while in the experimental group from 80 to 126 g. The largest juveniles in the control and experimental groups were analyzed. The percentage of juveniles weighing more than 110 g was 42% and 6% in the experimental and control group, respectively (Fig. 2).
The nutrient content in the feed affects the metabolism of fish, which determines the intensity of their growth and evolvement, and then the quality of commercial products. Proteins and fats are some of the main constituents of animal tissues and play an important energy role in metabolism. Lipids, in addition to energy, perform a number of vital functions in the fish body: structure-forming, regulatory, etc. Besides, they serve as precursors of many biologically active substances, including hormones (Tocher, 2003). The introduction of components that are not typical for the natural diet of rainbow trout can have significant impact on the metabolism of fish and, as a consequence, can lead to changes in their physiological state and growth processes.
The physiological state of trout underyearlings was assessed by the general chemical composition of the body.
A significantly (p≤0.001) higher level of moisture was observed in the muscles of the fish of the control group. The experimental group had the best results in terms of dry matter content in muscle tissue, which was 32.24% (p≤0.001). A slightly lower dry matter of 28.68% was found in fish feed pelleted food without the probiotic.
According to the results of the chemical composition of muscle tissue, the fish in the experimental group contained a slightly higher amount of protein and fat in the muscles. The protein content was 17.54%, which exceeded the fish in the control pond by 1.33% (p≤0.001), and the fat content was 13.9%, which was larger than the control group by 2.1%. The chemical composition of the muscle tissue of the studied trout is presented in Table 3.
Lactobacilli and Enterococci play an essential role in the trout GIT microbiota (Iorizzo et al., 2021; Lauková et al., 2019), and spore-forming bacilli were reported by many groups as potent probiotics for aquaculture (Nikiforov-Nikishin et al., 2021), which also justified our selection of the strain from our collection of probiotics. At the same time, Escherichia coli and Vibrio spp., while being fish commensal microorganisms, are raising concerns because of the pathogenicity of their representatives (for instance, Mothersill et al., 2015), especially when it comes to the possible transfer of antibiotic resistance genes in various environments, including in aquaculture (Capkin et al., 2015). Abundance of different microbial groups in the intestines of fishes fed probiotic and control are show in Table 4.
Generally, Lactobacilli, Enterococcus, Vibrio, Bacillus, and coliform bacteria were found in the intestinal samples of rainbow trout. There were no significant differences between the samples of the experimental and control groups, except for the number of Bacillus bacteria.
In the case of Bacillus in the control group, 5.0±0.4×103 CFU/g of bacteria with different colony morphology was found. However, in the experimental group, the number of Bacillus representatives was higher (8.4±0.8×104 CFU/g).
In general, the data indicate that the probiotic bacteria B. amyloliquefaciens B-1895 has no negative impact on the selected microorganisms in the studied fish. Future research is needed to understand the possible influence of the studied probiotic on the trout’s microbiome, especially considering reports pointing at the ability of certain probiotic formulations to modulate the juvenile trout’s microbiome (Gonçalves and Gallardo‐Escárate, 2017). Probiotic B. amyloliquefaciens B-1895 has discernible biological activity when supplemented into the diet of fish. The higher weight gain in trout of the experimental group is likely due to the effect of the probiotic, which affects the increase in the total number of intestinal bacteria and the proportion of bacteria that break down starch (Zuenko et al., 2017). Probiotics can improve feed digestibility, increase the net availability of essential nutrients, and improve the immunity and intestinal health of host animals (Markowiak and Śliżewska, 2018; Yirga, 2015).
At the same time, the weight of the fish and the feed digestibility increased. The analysis of the results of the biochemical parameters of the muscle tissue of trout indicates the normal physiological state of all experimental fish.
Thus, the observed increase in the quantity of protein and fat in the muscle tissue of the probiotic-fed fish confirms the obtained results on the stimulation of their mass accumulation and digestive activity (Ostroumova, 2012). The growth indices testify to the positive effect of the probiotic introduced in the mixed feed on the juvenile trout.
The obtained results give reason to assert that the feed with probiotic preparation on growth, survival rate, and health of fish is highly effective, which is confirmed by the chemical composition of the muscle tissue and gut microbiota of rainbow trout.
Conclusion
The positive effect on trout fry was expressed in an increase in the conversion of feed containing a probiotic supplement, an increase in growth, survival, and body weight. The use of a probiotic ensured the normal physiological state of the fish. Increasing the proportion of protein and fat promoted muscle tissue build up in the fish. The biological and physiological data obtained in the course of the conducted research allow us to recommend the introduction of a probiotic supplement in the amount of 0.15% of the feed weight into the commercial feed for rainbow trout. The amount of introduced probiotic is conditioned by biological needs of the organism and activity of the bacterial preparation.