Introduction
Lactiplantibacillus plantarum is a commensal microorganism in the human gastrointestinal (GI) tract and is also commonly found in many fermented foods (Kaushik et al., 2009). Owing to its long history of safe use, L. plantarum has been acknowledged as “Generally Regarded as Safe (GRAS)” by the United States Food and Drug Administration (FDA) and as suitable for the “Qualified Presumption of Safety” by the European Food and Safety Authority (EFSA; Liu et al., 2018).
A variety of health-beneficial effects of L. plantarum have been previously demonstrated. The administration of L. plantarum FBT215 significantly lowered the Firmicutes/Bacteroidetes ratio in healthy mice and effectively alleviated colonic inflammation (Chang et al., 2021; Lee et al., 2023). L. plantarum 200655 decreased oxidative stress in HT-29 cells and improved the texture attributes of yogurt such as water-holding capacity and viscosity (Kariyawasam et al., 2023). D-Galactose-mediated oxidative stress was suppressed while hepatic glutathione peroxidase activity was promoted in aged mice with the administration of L. plantarum C88 (Li et al., 2012). Some L. plantarum strains, such as L. plantarum TWK10 and L. plantarum HY7715, improved muscle mass and exercise performance in a rodent model, but details underlying the mechanism for attenuation of sarcopenia are still not certain (Chen et al., 2016; Lee et al., 2021). The authors postulated that L. plantarum HY7715 might contribute to improving the rate of protein digestion and absorption.
Whey proteins are side-stream products of cheese manufacture and are highly availability as a dietary protein source. Whey proteins contain a high concentration of branched-chain amino acids (BCAA: Leu, Ile, and Val, 22.3%) which play an important role in skeletal muscle synthesis compared to casein (20.3%), soy (17.5%), and wheat protein (14.1%) (Morifuji et al., 2009). According to Park et al. (2018), protein supplementation improves muscle mass and physical performance in malnourished older adults, but its efficacy depends on the level and type of protein. They reported that protein supplementation at a level of 1.5 g/kg/day provided a beneficial effect on the prevention of sarcopenia. The supplementation of hydrolyzed whey protein isolate showed greater improvement in lean muscle mass and muscle strength during 10 wks of resistance training in bodybuilders compared to caseins (Cribb et al., 2006). These results suggest that hydrolyzed low-molecular-weight peptides containing Leu possibly accelerate the utilization of amino acids for muscle synthesis compared to intact whey proteins.
The proteolytic activity of lactic cultures plays an important role in flavor and texture development and the liberation of bioactive peptides in the production of fermented foods (Satılmış et al., 2023). Kim et al. (2023) reported that the administration of milk protein and a probiotic strain with high proteolytic activity significantly improved the digestibility of proteins in mice. This suggests that a combination of protein and probiotic culture might improve the transport of amino acids for muscle synthesis.
The objective of this study was to screen potential probiotic strains for their ability to improve the bioavailability of BCAA in whey proteins for muscle synthesis. To achieve this goal, a promising L. plantarum strain was selected based on digestibility and BCAA production from whey proteins, and the effect of the selected probiotic strain (L. plantarum LM1001) on myogenesis was evaluated using C2C12 myoblasts.
Materials and Methods
L. plantarum LM1001 was isolated from kimchi, Korean fermented vegetables, as previously described (Bae et al., 2022). Briefly, homogenized kimchi was diluted in phosphate-buffered saline (PBS) and plated on de Man-Rogosa-Sharpe agar (MRS agar; BD Difco, Franklin Lakes, NJ, USA). To isolate Lactobacillus species, the colonies isolated from MRS agar were spread on bromocresol purple (BCP) containing MRS agar. Yellow colonies on BCP agar plate were cultured again in MRS agar. For the characterization of the isolated strain, a single purified colony was enriched in MRS broth and further analyzed by 16S rRNA gene sequencing. The identified Gram-positive and catalase-negative L. plantarum strain was named LM1001.
The medium was prepared by dissolving 50 g of whey protein concentrate (WPC; Marquez Brothers International, Hanford, CA, USA), 5 g of tryptone (Gibco, Paisley, UK), 2.5 g of yeast extract (BD Difco, Detroit, MI, USA), and 1 g of glucose (Daejung Chemicals, Siheung, Korea) in 1 L of distilled water. The medium was sterilized in an autoclave at 95°C for 10 min. To assess proteolytic activity, L. plantarum strains were cultured in MRS and harvested by centrifugation (13,572×g, 4°C, 10 min) and washed twice with PBS. The washed bacteria were resuspended in PBS to approximately 10 Log CFU/mL and 1% (v/v) of the suspension was inoculated into WPC medium (the final concentration of L. plantarum strain was adjusted to 8 Log CFU/mL). The same volume of PBS was used instead of resuspended bacteria as a control. The mixture was incubated at 37°C for 48 h under shaking at low speed. The protein concentration of initial WPC and cultured WPC was measured by the bicinchoninic acid (BCA) assay (Smith et al., 1985). The ratio of WPC degradation was calculated as follows:
Where, A0 is the initial protein concentration of WPC medium, and A48h is the protein concentration of WPC medium after 48 h incubation. L. plantarum ATCC14917 (type strain) was used as a control.
After incubation of WPC medium in the presence of L. plantarum LM1001, the culture supernatant was obtained by centrifugation (15,928×g, 4°C, 10 min). The supernatant was collected, filtered through a 0.22 μm syringe filter (Adventec, Tokyo, Japan) and named LP-WPC. LP-WPC was stored at −20°C until use.
The genomic DNA of L. plantarum LM1001 was extracted using the TaKaRa MiniBEST Bacteria Genomic DNA Extraction Kit (Takara Bio, Kusatsu, Japan). The DNA sequencing library was constructed using single molecular real-time sequencing technology (Pacific Biosciences, Menlo Park, CA, USA). The hierarchical genome assembly process for de novo assembly was performed using the Celera Assembler (Macrogen, Seoul, Korea). In order to compare the proteolytic genes of L. plantarum LM1001 with those of L. plantarum ATCC 14917, as the reference strain, complete genome sequences of both strains were obtained from the NCBI microbial genome database (https://www.ncbi.nlm.nih.gov/genome).
The free amino acid content of LP-WPC was determined by the method of Tang et al. (2023) with a slight modification. Briefly, an equal volume of trichloroacetic acid (TCA) solution (10%, v/v) was added to LP-WPC. The mixture was placed at room temperature for 60 min and centrifuged at 18,472×g for 10 min. The supernatant was filtered through a 0.22 μm syringe filter (Adventec) prior to HPLC analysis. An Agilent 1260 Infinity HPLC system equipped with a diode array detector (Agilent, Santa Clara, CA, USA) was used for analysis. The separation was carried out by means of an Agilent Zorbax Eclipse AAA column (4.6 mm×150 mm, 3.5 μm) using a mobile phase consisting of 0.1% formic acid in water (v/v) as mobile phase A and 0.1% formic acid in acetonitrile (v/v) as mobile phase B. Gradient elution was performed as follows: 0 min, 10% B; 1 min, 20% B; 10 min, 40% B; 40 min, 50% B; 42 min, 100% B; 57 min, 100% B; 60 min, 90% B; 70 min, 10% B. The flow rate was set to 0.4 mL/min, and the column oven temperature was maintained at 40°C. The UV detection was performed at 338 nm. The quantification of amino acid was performed using the external standard. Each sample was analyzed in triplicate with three independent determinations.
The probiotic properties of L. plantarum LM1001 including its resistance to gastric and bile salt conditions, adhesion to intestinal epithelial cells, and auto-aggregation were measured as described previously (Bae et al., 2023). Briefly, cultured L. plantarum LM1001 was incubated at 37°C either in MRS broth containing pepsin (0.3%, pH 2.5, 2 h) or oxgall (0.3%, 24 h) and the viable cells were counted by spread plate method on MRS agar. For adhesion test, HT-29 intestinal epithelial cells (ATCC, Manassas, VA, USA) were harvested at 80% confluence, and the harvested cells were seeded (1×105 cells/well) and incubated to form a monolayer. The HT-29 monolayer was treated with L. plantarum LM1001 (8 Log CFU/mL) for 2 h without antibiotics. After washing nonadherent bacteria with PBS, and viable cells were counted. In the auto-aggregation test, cultured L. plantarum LM1001 was washed with PBS and adjusted to have an A600 of 0.5. After incubated at 37°C for 24 h, the absorbance of upper suspension was measured at 600 nm. The aggregation (%) was calculated as follows:
Where A0h is the initial absorbance at 600 nm, and A24h is the absorbance at 600 nm after 24 h.
The intrinsic enzyme-producing activities of L. plantarum LM1001 were determined using the API ZYM kit (BioMérieux, Marcy-l'Etoile, France) according to the manufacturer’s guidelines.
C2C12 mouse myoblasts were obtained from the American Type Culture Collection (ATCC). The cells were maintained in complete Dulbecco’s modified Eagle medium (DMEM; Welgene, Daegu, Korea), which contains 10% fetal bovine serum (FBS, Welgene) and 1% penicillin-streptomycin (Welgene), at 37°C in a 5% CO2-humidified incubator. To induce myogenic differentiation, C2C12 cells (5×104 cells/well) were plated in a 6-well plate and cultured for 3 days in complete medium. When the cells reached 80% to 90% confluency, the medium was switched to differentiation medium containing 2% horse serum (MB Cell, Kisanbio, Seoul, Korea) and 1% antibiotics to induce myotube differentiation. LP-WPC (1%, 2%, and 3%) was treated to C2C12 cells, and the effect on the expression of myogenic regulatory factors, such as myogenic factor 5 protein (Myf-5; Abcam, Cambridge, UK), myoblast determination factor 1 (MyoD; Santa Cruz Biotechnology, Santa Cruz, CA, USA), myogenin (Abcam), was analyzed using western blotting. Cytotoxicity of LP-WPC on C2C12 cells was monitored, and western blotting was conducted, as previously described (Bae et al., 2022).
Statistical analyses were performed using SPSS Statistics version 21 software (IBM, Armonk, NY, USA). When a significant difference (p<0.05) was found in the analysis of variance (ANOVA), Duncan’s multiple comparison and Student’s t-test were conducted to determine the significant difference between treatment means.
Results and Discussion
To analyze the proteolytic activity, various L. plantarum strains were cultured in WPC medium, and changes in protein concentration were compared. As shown in Fig. 1, five strains resulted in a significant decrease in protein content after incubation (p<0.05, Fig. 1A). L. plantarum LM1001 strain displayed the greatest proteolytic activity among the tested strains (Fig. 1B).
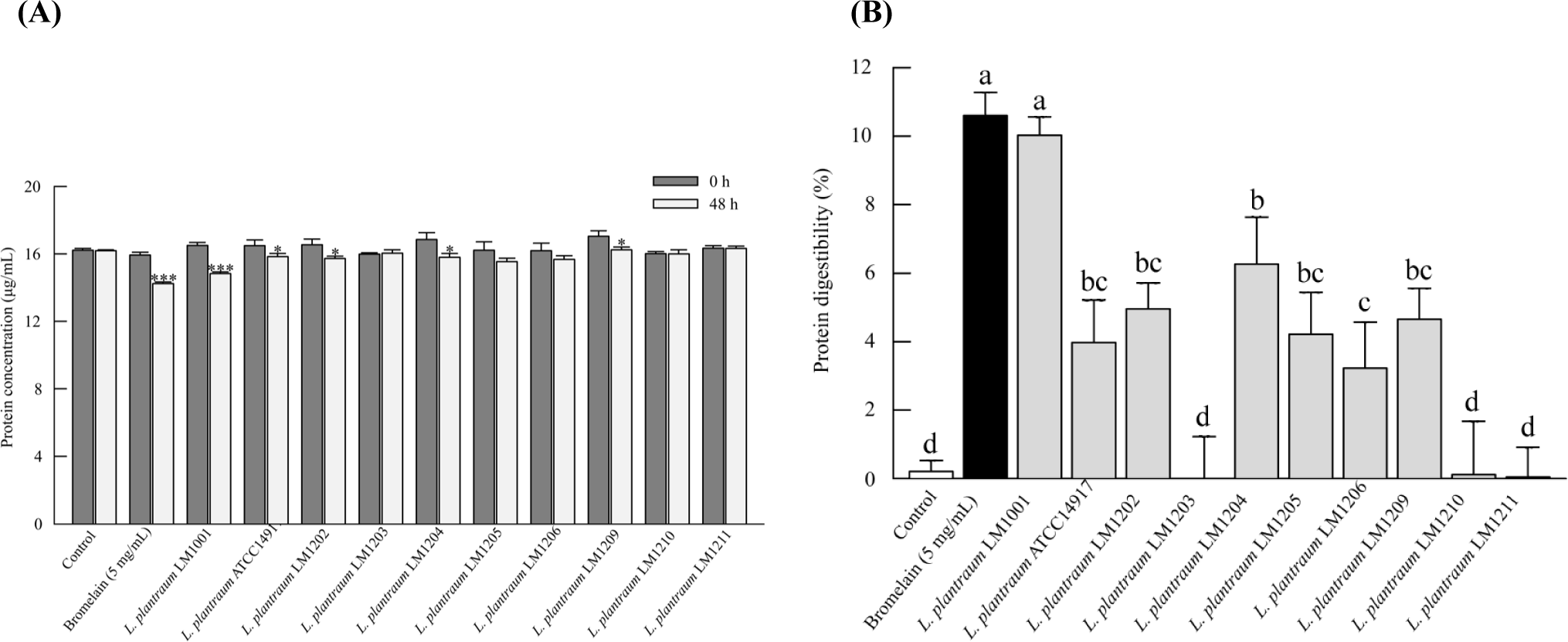
There is no standard method for the evaluation of proteolytic activity of lactic acid bacteria strains, although the agar-well diffusion test has often been used as an index of proteolytic activity (Beganović et al., 2013). However, the measurement of a clear zone diameter is not accurate enough as a quantitative assay for the determination of proteolytic activity. The BCA assay is a total protein assay. The principle of the assay is based on the reductive properties of the peptide bonds to reduce Cu2+ to Cu+ under alkaline conditions. When the Cu+ reacts with the organic dye, BCA, a purple complex is formed that can be measured spectrophotometrically at 562 nm. The extent of reduction of Cu2+ is also dependent on the protein composition, such as Trp, Tyr, Cys, and cystine (Smith et al., 1985). Wiechelman et al. (1988) reported that at least a tripeptide is required for complex formation between Cu+ and BCA. Thus, the decreased color intensity in the BCA assay was probably due to proteolysis-mediated peptide bond reduction, as the total moles of oxidizable amino acids in the substrate (WPC) are the same. The protein content was consistently decreased by the addition of bromelain, a positive protease control (Fig. 1A).
Whey protein is an excellent dietary protein source for muscle synthesis and the prevention of sarcopenia (Devries and Phillips, 2015). The interplay between the host and gut microbiota influences protein metabolism by modifying metabolite production and amino acid homeostasis (Lin et al., 2017). Probiotics indirectly affect protein metabolism by altering the gut microbiota composition or directly improve protein digestion by promoting digestive enzyme activity in the gut (Wang and Ji, 2019). It has been demonstrated that the intake of protein together with probiotics with high proteolytic activity promoted digestibility and bioavailability of proteins (Jeon et al., 2023).
The proteolytic and peptidolytic activity varies depending on the bacterial species and strains. Table 1 shows the results of the whole genome sequencing (WGS) analysis of L. plantarum LM1001. The number of the protease- and peptidase-related genes of L. plantarum LM1001 was 81, which was about twice as many as that of L. plantarum ATCC 14917, the type strain of L. plantarum species. The high proteolytic genetic potential of L. plantarum LM1001 might be related to its superior proteolytic activity to other L. plantarum strains. In particular, L. plantarum LM1001 has 19 metalloprotease or metallopeptidase (MMP)-related genes containing one or two divalent metal ions, such as Zn2+, Ca2+, Mg2+, and Cu2+ in active sites (Hasan et al., 2021). Based on these results, L. plantarum LM1001 was selected as a strong WPC hydrolytic strain and used for further study. In addition, proteolytic activity of lactic acid bacteria varied depending on the type of protein such as soy protein, casein, and whey protein. L. plantarum LM1001 showed strong proteolytic activity especially on WPC (data not shown).
Changes in total free amino acids and BCAA content in the WPC culture supernatant were determined after TCA (10%, v/v) precipitation of LP-WPC. As shown in Table 2, the total free amino acids and BCAA content were significantly increased by fermentation with various L. plantarum strains. This implies that proteolysis of protein molecules occurred by proteases/peptidases released from L. plantarum strains. LMW peptides and free amino acids generated by the metabolism and transformation of intact dietary protein in the gut are readily absorbed by the gut endothelial cells and improve the bioavailability of WPC.
BCAA plays an important role in muscle biosynthesis. In particular, Leu promotes protein synthesis through the activation of mechanistic target of rapamycin complex 1 (mTORC1; De Bandt, 2016). Leu supplementation improved the regeneration of skeletal muscles, decreased the inflammation area, and increased the number of proliferating satellite cells in muscles from old rats (Pereira et al., 2015). The concentration of BCAA (including Leu) in the culture supernatant was significantly increased by fermentation, and it varied among the L. plantarum strains. The cultures fermented with L. plantarum LM1001 showed the highest total free amino acids (4,261 μg/mL) and BCAA (2,208 μg/mL) content. L. plantarum LM1206 and L. plantarum LM1205 strains also produced high levels of free amino acids but they were similar to those of L. plantarum ATCC 14917, the type strain.
L. plantarum strains displayed different proteolytic characteristics and provided different bioactivities and sensory impacts depending on the amino acid profile produced (Satılmış et al., 2023). Chu et al. (2023) demonstrated that the administration of L. plantarum CCFM405 alleviated Rotenone-induced Parkinson’s disease by modulating gut microbiota and BCAA biosynthesis. This suggests that administration of appropriate probiotics plays an important role in the generation of desirable metabolites such as BCAA.
The probiotic properties of L. plantarum LM1001, including its resistance to pepsin and bile salt, adhesion to HT-29 cells, and auto-aggregation, were evaluated. As shown in Table 3, L. plantarum LM1001 showed more than 99% acid tolerance and 73% bile salt tolerance, respectively. The tolerance of L. plantarum LM1001 to bile salt was significantly higher than that of L. plantarum ATCC 14917 (p<0.05), whereas the adherence ability to HT-29 cells and auto-aggregation (%) of L. plantarum were comparable to those of the L. plantarum type strain.
Probiotics are live microorganisms that contribute to host health, and the abilities to survive and colonize in the GI tract environment are some of the major criteria for the selection of probiotics (Pramanik et al., 2023). The attachment of Lactobacillus spp. to human enterocytes occurs through the cell surface components of microorganisms such as cell-surface collagen-binding proteins and cell wall-anchored proteins (CWAP; Bae et al., 2023). CWAP contains an amino acid motif consisting Leu-Pro-X-Thr-Gly (LPXTG, X: any amino acid), and cleavage of the Thr residue by sortase (Srt) enzymes mediates cell wall attachment to human epithelial cells (Zhang et al., 2015). LPXTG and Srt-related genes were identified in the WGS analysis of L. plantarum LM1001, and these genes could contribute to the adhesion of probiotics.
Probiotic strains produce enzymes, which influence the utilization of nutrients. Table 4 indicates the intrinsic enzyme-producing activities of L. plantarum LM1001. L. plantarum LM1001 showed 13 enzyme activities, such as alkaline phosphatase, esterase, esterase lipase, leucine arylamidase, and β-galactosidase. Among these enzyme activities, β-galactosidase activity can alleviate lactose intolerance by catalyzing the hydrolysis of lactose into glucose and galactose. Whey protein powder for muscle growth is typically produced from WPC and contains 15% lactose (dry weight basis). The combined ingestion of whey protein powder and L. plantarum LM1001 might relieve discomfort derived from lactose maldigestion. In addition, L. plantarum LM1001 did not display β-glucuronidase activity which alters xenobiotic availability in the gut and is associated with an increase in the risk of colon cancer (Sears and Garrett, 2014).
Consistent with the results of the present study, L.plantarum Ln4 and L.plantarum G72 strains displayed β-galactosidase activity and did not produce β-glucuronidase, whose activity is harmful to human health (Son et al., 2017). The genes encoding β-galactosidase and glycoside hydrolase were also identified in the WGS analysis (β-galactosidase, β-galactosidase small subunit, glycosidase hydrolase family 25, family 78 glycoside hydrolase catalytic domain, and glycoside hydrolase family 1, 13, 65, and 73 proteins).
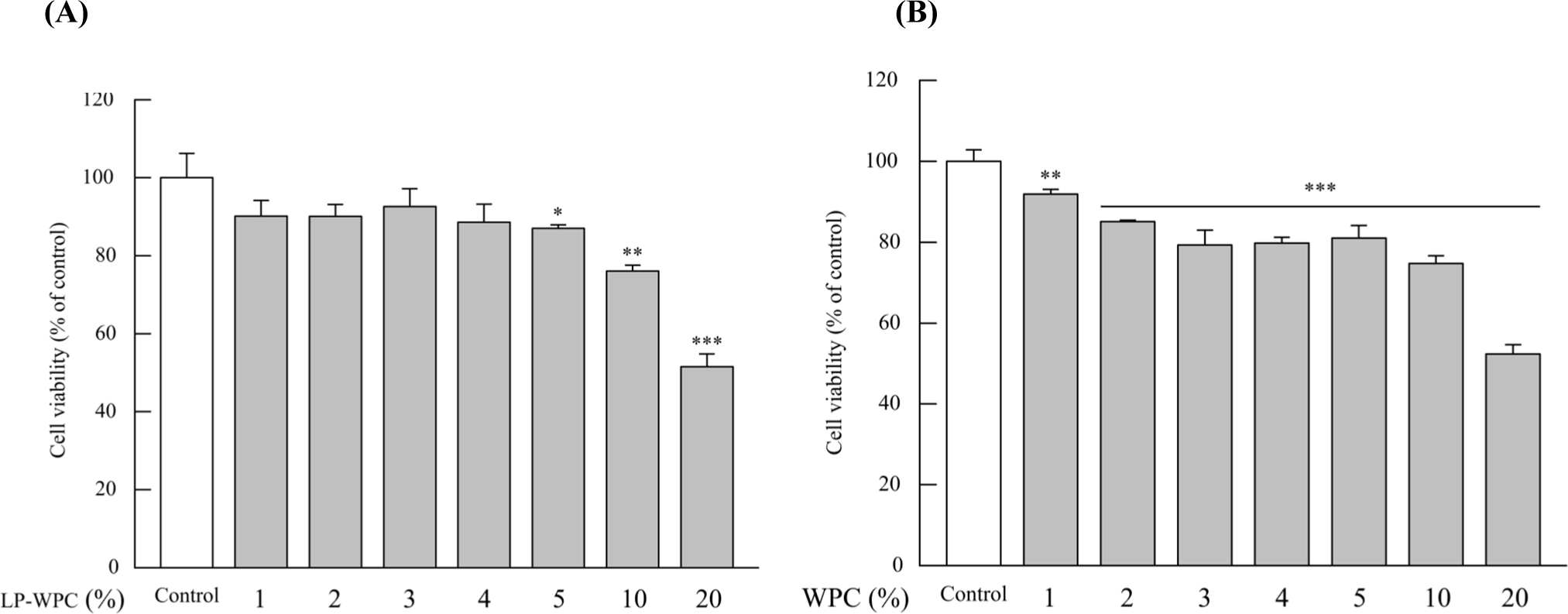
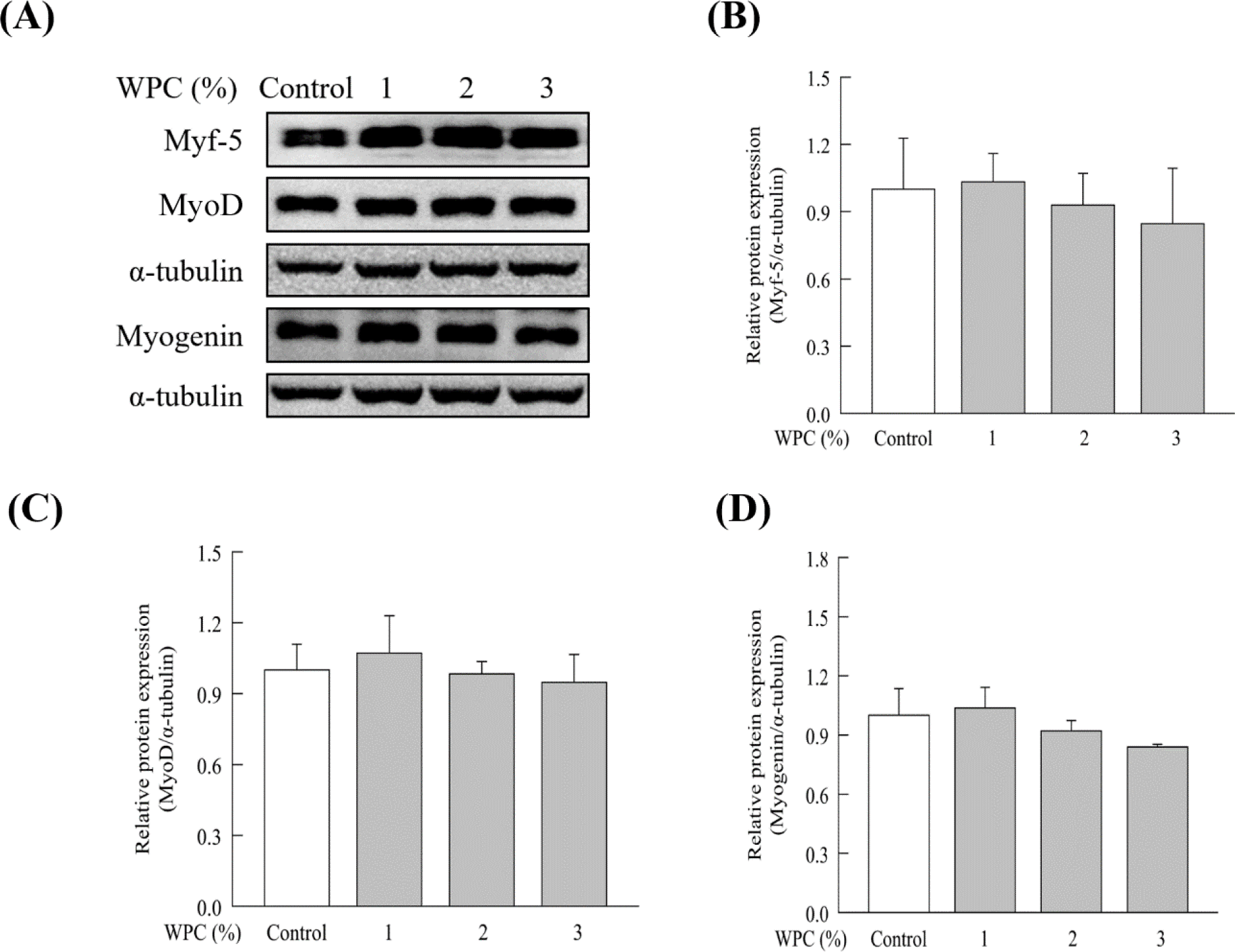
The effect of LP-WPC on the differentiation of skeletal muscle cells was determined using C2C12 myoblasts. As shown in Fig. 2, the treatment with more than 5% sample (WPC vs. LP-WPC culture broth) showed less than 80% cell viability in the MTT assay. Thus, the non-cytotoxic concentrations of the sample were selected as 1%, 2%, and 3%. LP-WPC treatment at the level of 2% and 3% of medium significantly upregulated the protein expression of all myogenic regulator factors, such as Myf-5, MyoD, and myogenin in C2C12 cells (p<0.05, Fig. 3). Conversely, there were no significant changes in the expression of myogenic factors by treatment with WPC broth (Fig. 4). These results suggest that LP-WPC can enhance myogenic activity in myoblasts.
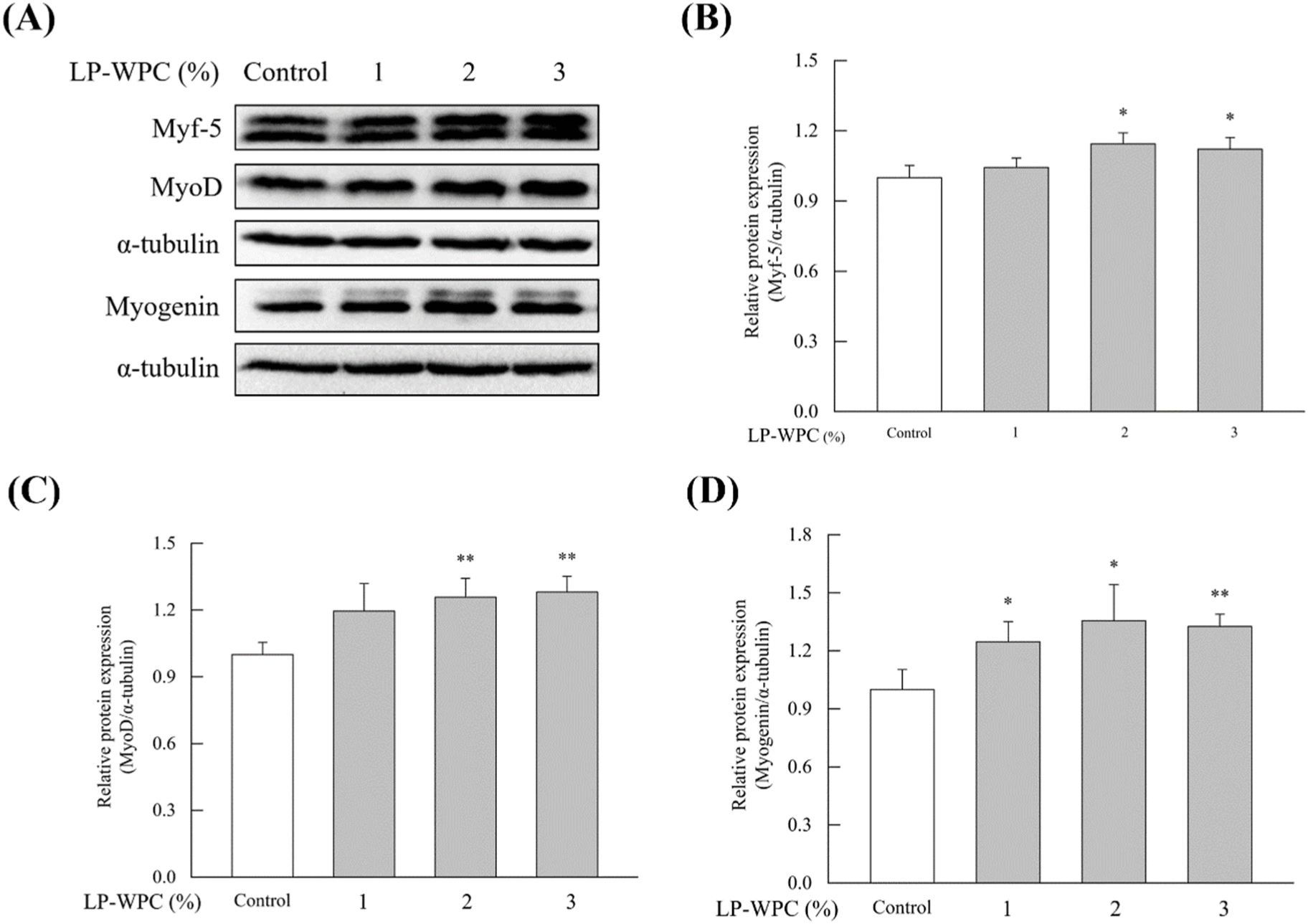
Myogenesis is a process that regulates the complex growth and maturation of muscle tissue, including the proliferation, differentiation and maturation of myoblasts (Allen et al., 1979). Myoblasts with a single nucleus are fused to form multinucleated structures, and MyoD and Myf-5 play pivotal roles in myoblast commitment and myogenic differentiation (Sabourin and Rudnicki, 2000). Myogenin contributes to muscle homeostasis as a secondary myogenic regulator. Mutation of myog in zebrafish caused a decrease in muscle mass and muscle fiber size (Ganassi et al., 2018).
The increased expression of myogenic regulators in response to LP-WPC is probably due to the generation of BCAA in the culture supernatant. The supplementation of BCAA promoted mTOR1 signaling and simultaneously activated the autophagy function of muscle cells in patients with liver disease (Tsien et al., 2015). The activation of mTORC1 signaling is regulated by various factors such as mechanical and endocrine stimuli, intercellular energy level, and the availability of amino acids (Bar-Peled and Sabatini, 2014). Atherton et al. (2009) examined the effect of essential amino acid (EAA) on protein synthesis in C2C12 skeletal muscle cells and found that Leu significantly promoted mTOR and 4E-binding protein 1 (4EBP1) signaling, but other EAA had no effect on anabolic signaling.
Conclusion
Adequate protein consumption and physical exercise are major strategies to improve muscle mass. WPC is widely used in protein powder formulations. L. plantarum LM1001 was selected based on the digestibility and potential to generate BCAA from WPC. The addition of LP-WPC to C2C12 myoblasts significantly increased the expression of Myf-5, MyoD, and myogenin reflecting a promotion in the formation of myotubes. L. plantarum LM1001 displayed β-galactosidase activity but did not produce β-glucuronidase. Thus, the intake of whey protein together with L. plantarum LM1001 has the potential to aid protein digestion and utilization. The effect of this combination on muscle mass in an animal model is currently underway.













