Introduction
The use of binders is essential for emulsified meat products (including frankfurters, sausages, and bologna) and reconstituted meat products (including reconstituted hams, patties, and steaks; Herz et al., 2023) as it can promote high production and cost-effectiveness of meat products, while meeting textural characteristics and consumer preferences (Dekkers et al., 2018). Therefore, producers in the meat processing industry use various non-meat binders (NMBs; protein-based, carbohydrate-based) such as salt, phosphate, transglutaminase, and protein to promote water holding capacity (Owusu-Ansah et al., 2022). Soluble proteins extracted by salt play an important role as binders in meat products (Herz et al., 2021). However, the addition of large amounts of salt can reduce the food appeal of meat products. In addition, it is not sufficient to produce a complete product in the food industry. As a result, the use of binders is necessary to increase the binding force of meat products.
Recently, safety issues related to chemical additives used in meat products have emerged. Consumers demand safe and nutritious meat products that are tasty, healthy, and functional (Tahmasebi et al., 2016). Therefore, research is being conducted to develop products using natural substances in meat processing. Frequently used natural NMBs are either protein-based or carbohydrate-based (Guedes-Oliveira et al., 2021). There are a variety of NMBs, including soy proteins, milk proteins, gluten proteins, plasma proteins, egg proteins, gelatin, hydrocolloids, dietary fiber, sugars, and starches (Lu et al., 2021; Nasrollahzadeh et al., 2021). These proteins have the functional property of retaining moisture and fat mainly due to hydrophilic (water-loving) and lipophilic (fat-loving) groups of protein molecules and their hydrogen bonds (Vilgis, 2023).
The efficacy of various NMBs, depending on their amount and type, can affect the binding capacity, emulsion capacity, lipid oxidation, microbiology, and nutritional value of meat products (Anzani et al., 2020; Reddy et al., 2023). Animal plasma proteins contain high molecular weight compounds such as albumin, globulin, and fibrinogen with diverse functionalities. They are mainly used as emulsifiers, stabilizers, colorants, fertilizers, or pharmaceuticals (Toldrá et al., 2021). White egg powder (EWP), soy protein isolate, and sodium caseinate (SC) are the three most commonly used proteins in the meat processing industry. Seaweed contains polysaccharides, proteins, and essential fatty acids, especially alginate, the most abundant ionic polysaccharide (Ferrara, 2020).
Research is being conducted on how each NMB affects emulsified sausages. However, there is a lack of research on comparisons between various binders and how they affect storage properties. Therefore, this study investigated effects of adding NMBs on physicochemical and storage properties of pork emulsified sausage.
Materials and Methods
Blood samples from cattle and pigs were collected immediately after slaughter and used directly for plasma sampling. Blood samples were immediately transported to the laboratory and stored on ice. It was prepared by adding and mixing ethylenediaminetetraacetic acid at a rate of 2 g/L to the collected blood. Bloods were centrifuged at 8,000×g and 15 min at 4°C using a refrigerated centrifuge (SUPRA 25K, Hanil Science, Gimpo, Korea). The separated plasma was completely frozen at –96°C using a freeze dryer (PVTFD10R, Ilshinlab, Dongducheon, Korea). Bovine blood plasma (BBP) and porcine blood plasma (PBP) powders were ground to a certain size. The EWP, commercial porcine plasma powder (CPPP), isolated soy protein (ISP), seaweed powder (SP), and SC were purchased DONG BANG Food Master (Eumseong, Korea) and used in the experiment.
Fresh pork lean Biceps femoris meat and back-fat from LYD (Landrac×Yorkshire×Duroc) pork were purchased from a local market. Pork meat and back-fat were ground twice through a 5-mm plate. Experimental groups included Control (Non-addition), BBP (1% BBP); CPPP (1% CPPP), PBP (1% PP), T4 (1% EWP), SP (1% SP), ISP (1% ISP), and SC (1% SC), respective treatments were prepared (Table 1). The formular consisted of 70% meat, 15% back fat, and 15% ice water. Salt-soluble proteins were extracted from minced meat with 1% salt for 1 min using a bowl cutter (Talsa K30, DSL Food Machinery, Valencia, Spain). Next, 0.4% sugar, 1.2% mixed seasoning, NMBs, and half of ice were added and mixed for 2 min, at this time, the emulsion batter temperature is below 10°C. The emulsion was then filled into a fibrous casing (Nalo Top, Kalle, Wiesbaden, Germany, diameter 70 mm) using a stuffer (IS-8, Sirman, Padova, Italy). The stuffed emulsion samples were heated in a heating chamber (Thematec Food Industry, Seongnam, Korea) to reach an internal temperature of 75°C, and the manufactured sausages were immediately refrigerated and stored for 5 weeks at 4°C before conducting the experiment.
Approximate compositions of both treatments were determined using the analytical method of AOAC (2016a), AOAC (2016b), AOAC (2016c), and AOAC (2016d).
The weight measured after filling the casing with the emulsion batter was referred to as the initial weight and was then heated to an internal temperature of 75°C. The heated sausages were then cooled for 30 min at 4°C and weighed (final weight). Cooking yield was then calculated using the following formula:
In this experiment, volatile basic nitrogen (VBN) was measured using a modification of Pearson (1976). To 10 mL of sample (mixed with distilled water), a few drops of 0.5 wt% phenolphthalein indicator (dissolved in 50 wt% ethanol) and 3.5 mL of 20% sodium hydroxide were mixed. The 250 mL of distillate was collected in the flask, which was immediately sealed and collected. The distillate was collected in a flask containing 20 mL of 4% boric acid and Tashiro indicator (methyl red: methylene blue=2:1). Afterwards, the obtained basic solution (green) was titrated with 0.01M hydrochloric acid until it turned gray. The VBN content was measured after correction: the blank was measured by steam distillation of 6% perchloric acid.
The 5 g of each sample and 15 mL of deionized distilled water were homogenized for 10 s at 3,000×g using a homogenizer (CPPP5, IKA Werke, Staufen, Germany). Afterwards, butylated hydroxyanisole (50 μL, 10%) and thiobarbituric acid/ trichloroacetic acid (TBA/TCA; 2 mL) were added to 1 mL of the homogenate and mixed. The mixture was reacted in a water bath at 100°C for 15 min to develop color. The colored sample was immediately cooled for 10 min to bring it to room temperature, and then centrifuged at 2,000×g for 15 min to obtain the supernatant. The 1 mL of distilled water and 2 mL of TBA/TCA solution were used as blanks, and absorbance was measured at 531 nm. The amount of 2-thiobabituric acid reactive substance (TBARS) was then expressed as milligrams of malondialdehyde per kilogram of sample.
Free amino acids were tested transforming the method of Aristoy and Toldra (1991). Samples were extracted with 0.01N HCl, and 300 μL of the extracted sample was mixed with 10 μL internal standard (l-citrulline) and 690 μL acetonitrile. The mixture was reacted at 4°C for 30 min, and then centrifuged at 10,000×g for 15 min at 4°C using a centrifuge. The collected supernatant was filtered through a 0.45 μL membrane filter and used in the experiment. At this time, external standards (amino acid standard: 0.25 nM, Agilent, Santa Clara, CA, USA; glutamine, Sigma-Aldrich, St. Louis, MO, USA) were analyzed for O-phthalaldehyde and 9-fluorenylmethyl chloroformate derivatization using HPLC (Agilent; Herbert et al., 2000). Analysis conditions were conducted according to the method of Henderson et al. (2000), and the conditions were as follows: pH 7.8; Column, Zorbax Eclipse AAA, 4.6×60 mm, 5 μm; DAD detector, 262 nm, 338 nm; Column temperature, 40°C; Mobile phase A, 40 mM sodium phosphate buffer, and mobile phase B, acetonitrile:methanol:water, 45:45:10 (v:v:v).
There were a total of 144 samples used in the statistical analysis in the experiment, each consisting of 8 treatments×3 repetitions×2 storage times×3 batches. Batch means conducted at different times in the same place and temperature, and the experiment had a completely randomized design. Data on physicochemical and storage properties of sausages were subjected to one-way analysis of variance (ANOVA) using the general linear model procedure in the SAS program. Physicochemical properties were analyzed based on statistics of the treatment group. Storage characteristics were analyzed using the statistics of eight treatment groups and two storage time. Statistical significance between means was determined at the 95% significance level using Duncan’s multiple range test. Mean values and SD are presented. Correlation coefficients between storage parameters (pH, fat and protein contents, TBARS, and VBN) were tested using Pearson’s correlation using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results and Discussion
The influence of NMBs on approximate compositions of pork emulsified sausages is shown in Table 2. Moisture content was significantly higher in the control than in other groups. It was significantly higher in BBP and CPPP than in EWP and SP (p<0.05). According to Hsu and Sun (2006), sodium casein consists of 3.9% moisture, 91.7% protein, 0.7% fat, 3.6% ash, and 0.1% carbohydrate, while ISP contains 5%–6% moisture and 84.6% protein, 0.5%–1.0% fat, 4.0%–4.5% ash, and 3.9%–5.0% carbohydrate. EWP consists of 8.8% moisture, 80.2% protein, 0.2% fat, 5.1% ash, and 5.7% carbohydrate. Chemical compositions of dried seaweed are mainly dietary fiber and proteins (Premarathna et al., 2022). Plasma powder is composed mostly of protein. It contains 40%–50% globulin, 1%–3% fibrinogen, and 50%–60% albumin (Nair et al., 2022). Parés et al. (1998) have reported that approximate compositions of spray-dried porcine plasma are 66.45% protein, 14.13% ash, 11.83% moisture, and 3.88% fat. The plasma powder used in this study was lyophilized. Therefore, it was determined that pork sausages with added NMBs had higher solids content or lower moisture content than control sausages. Protein contents of sausages containing NMBs showed no significant difference between control and treatment groups. Fat contents of sausages added with EWP and SP were significantly higher than those of control sausages (p<0.05), which is believed to be due to the relatively low moisture content. These different values are thought to be due to different ingredients of the additive, as shown in the references mentioned above.
The influence of NMBs on heating yield of pork emulsified sausages is shown in Table 2. Most studies on NMBs added as meat binders to meat products have reported NMBs can reduce cooking yield and moisture loss while increasing emulsion stability, cohesion, water holding capacity, and hardness (Ismail et al., 2021; Ruther et al., 2020). Results of heating yield revealed that ISP, SP, and SC groups had higher values than the control. This meant that ISP and sodium casein contributed to the increase in thermal stability of frankfurter sausages due to formation of a protein network, consistent with previous research results (Yu et al., 2023). In addition, cooking losses of pork patties containing 1%–5% of seaweed (Laminaria japonica) were similar to previous research results showing that cooking loss of pork patties containing seaweed was significantly lower than that of the control due to the presence of alginate and laminarin in seaweed, which had water-holding and binding properties (Choi et al., 2012). Therefore, it was concluded that ingredients of NMBs such as ISP, SP, and sodium casein could affect product yields of emulsified pork sausages.
Effects of NMBs on amino acid compositions in emulsion type pork sausages are summarized in Table 3. Glutamic acid was known to contain a flavor not affected by the addition of NMBs (p>0.05). Among different sweet taste amino acids (serine, threonine, glycine, and alanine), significant difference was only observed in serine content. Serine contents of PBP, CPPP, ISP, and SC groups were higher than that of the control (all p<0.05). Contents of aromatic amino acids such as tyrosine and phenylalanine had no significant difference among treatments. Contents of amino acids (valine, phenylalanine, isoleucine, histidine, tyrosine, and arginine) with bitter taste had no significant difference among treatments. Contents of essential amino acids (threonine, valine, isoleucine, leucine, phenylalanine, histidine, lysine, and arginine) showed no significant difference either. Most amino acid compositions of pork sausages had no significant difference. This might be due to the fact that the NMB addition level was 1%, which might not be enough to affect amino acid compositions of sausages. Very few research studies have been conducted on amino acid compositions of sausages after adding NMBs. According to Márquez et al. (2005), isoleucine, lysine, and methionine contents in essential amino acids were not significantly different between bovine and porcine plasma proteins. Vilar et al. (2020) have reported that the addition of seaweed into frankfurter does not affect amino acid profiles. We also found that amino acid compositions of emulsion-type pork sausages were not influenced by the addition of 1% NMBs.
1) BBP, bovine blood plasma; PBP, porcine blood plasma; EWP, white egg powder; CPPP, commercial porcine plasma powder; ISP, isolated soy protein; SP, seaweed powder; SC, sodium casein.
4) BAA (bitter amino acid, valine, methionine, isoleucine, tyrosine, phenylalanine, histidine, arginine).
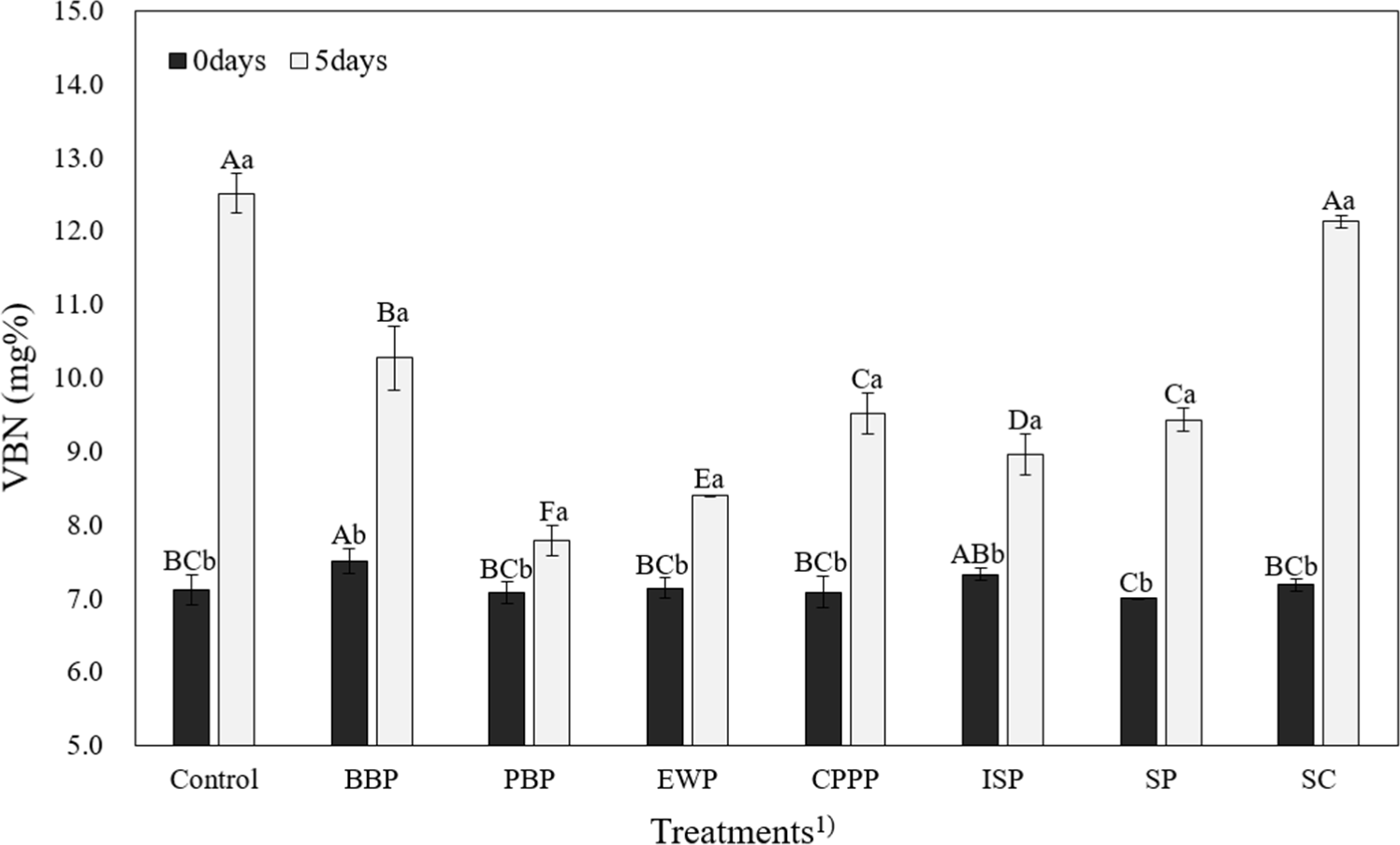
All VBN values of sausages increased significantly during five weeks of storage (p<0.05, Fig. 1). BBP and ISP had significantly higher VBN values than SP at week 0 (p<0.05). At the 5th week of storage, control and SC had significantly higher VBN values than other sausages (p<0.05). A high VBN value means that protein denaturation occurs during storage. It can be used as an indicator of the freshness of meat products (Lee et al., 2021). VBN values of all pork sausages, including the control group, did not exceed 20 mg% during the 5-week storage period (Cho et al., 2021). Therefore, it was believed that these NMBs did not have any adverse effect on the VBN of emulsion-type pork sausage during storage. These results suggest that EWP and SC, which have the lowest TBARS and VBN, play a positive role as NMBs.
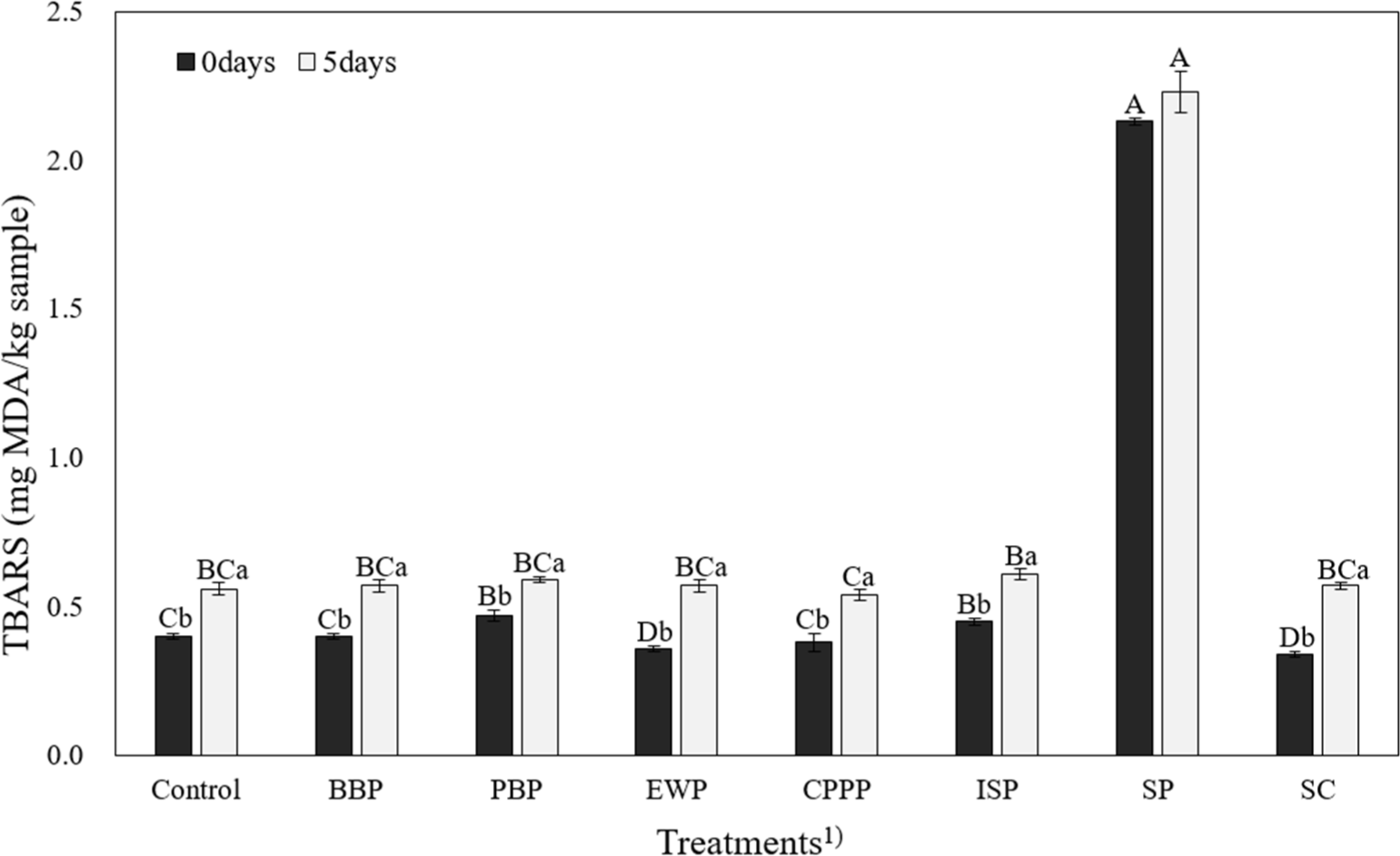
Effects of NMBs on TBARS values of emulsion-type pork sausages during refrigerated storage are shown in Fig. 2. TBARS values for other sausages were all less than 1.00 TBARS values of pork sausages at 0 and 5 weeks were the highest in SP among treatments (p<0.05). Most of the previous studies have reported that adding seaweed to food can reduce lipid oxidation (Harrysson et al., 2021; Munsu et al., 2021). Seaweeds contain polyphenols (e.g., phlorotannins) and carotenoid pigments (e.g., fucoxanthin) with the ability to scavenge free radicals, hydroxyl, and acetate radicals (Airanthi et al., 2011). Additionally, because seaweed has high K, Ca, Mg, and Mn contents, which might promote lipid oxidation (Teets and Were, 2008). Therefore, SP added to sausages in this study might have promoted oxidation. Additional research on this is needed. On the other hand, EWP and SC groups showed significantly lower lipid oxidation values than other treatments (p<0.05). It is believed that these binders might have structures that make it difficult for microorganisms to feed on.
Damage to meat products can be caused by several factors, and by analyzing this, the expiration date of food can be set and quality deterioration can be prevented (Hoa et al., 2023). Therefore, in this study, we attempted to analyze the correlation between indicators related to storage, which are shown in Table 4. It was found that pH showed strong negative correlations with protein and VBN (p<0.01), meaning that pH was more affected by protein than by fat in this study. Yang et al. (2020) have reported that protein and VBN are affected when protein additives are added to meat products. Therefore, an increase in pH due to the generation of ammonia from protein might have a greater effect during the storage period than the generation of acid due to fat rancidity (Bekhit et al., 2021). Fat showed a negative correlation with TBARS and protein showed a strong negative correlation with VBN (p<0.001). Therefore, when manufacturing emulsified sausages by adding NMBs, additional measures appear to be necessary to prevent protein spoilage.
| Traits | pH | Fat content | Protein content | TBARS | VBN |
|---|---|---|---|---|---|
| pH | 1 | –0.15 | –0.55** | –0.12 | 0.60** |
| Fat content | 1 | –0.26 | 0.61** | 0.11 | |
| Protein content | 1 | –0.06 | 0.71*** | ||
| TBARS | 1 | 0.18 | |||
| VBN | 1 |
Conclusion
In this study, effects of fattening protein binders on quality and storage characteristics of emulsified sausages were analyzed. There was no significant difference in protein content. In addition, fat contents of egg white power and SP were higher than that of the control. The difference in general ingredients is thought to be influenced by general ingredients of added additives. Seaweed, which contains a lot of dietary fiber, ISP, and sodium casein, which contains salt, appears to increase emulsion stability, resulting in high cooking yields in treatments containing these ingredients. The addition of NMBs did not affect amino acid compositions of sausages except serine. VBN showed a higher value at 5 weeks compared to 0 week, although did not exceed 20 mg% in any treatments. During five weeks of refrigerated storage, TBARS showed significantly higher values while egg white power and SP showed significantly lower values in SP more than other treatments. In conclusion, adding a NMB to tanned pork sausage can improve the binding force and develop healthier meat products that meet consumer demands. In this study, SP was found to be the most suitable natural binder.













