Introduction
Poultry meat is one of the most widely consumed meats worldwide, with no religious restrictions, in contrast to pork and beef, which are not consumed by Muslims and Hindus, respectively. Chicken is the most common type of poultry in the world. It contains less fat than the other types of meat and provides high-quality protein as well as other nutrients (Connolly et al., 2022). It can be processed in a variety of ways; nevertheless, the processing influences its customer acceptance (Maughan et al., 2012). Sensory perception of food encompasses aspects such as appearance, odor, flavor, taste, and texture; additionally, odor has a considerably strong effect on preference (Nanda et al., 2021). Food contains various volatile compounds (Ayseli et al., 2014), each of which has a unique olfactory profile that is determined by specific chemical combinations. The volatile compounds or flavors are mainly formed by degrading and reacting with non-volatile precursors such as fat, protein, peptides, fatty acids, amino acids, and reducing sugar (Madruga et al., 2010). As the processing and storage affect the reaction and the changes in the food compositions and then affect the volatile compound changes (Calkins and Hodgen, 2007), different heating conditions influence the products, giving them a specific aroma and bringing about changes in their volatile profiles during storage.
Boiling is a typical method for preparing or cooking chicken. Chicken is cooked in a variety of broths with varying flavors. The components change pH, which affects a variety of processes, including lipid oxidation and the Maillard reaction (Niu et al., 2016). Boiling at 95°C is a viable heating treatment for chicken, according to Wang et al. (2020b), because it helps to retain the fresh odor and texture while safeguarding food safety. This stands in contrast to the stewing method, which involves slowly heating the meat in the broth to liberate the fatty acids and amino acids and produce the desired flavors or volatiles (Guan et al., 2023). Boiled and pasteurized chicken meat has a shelf life of up to 7 d under mild chilling conditions with acceptability of flavor and odor (Montero-Prado and Morales, 2022) and customer safety (Hasani et al., 2023). There have not been many studies, as far as we know, on how pH affects the volatile flavor of cooked chicken after heat treatment. Therefore, the volatile components of chicken that were boiled at various pH levels as well as the volatile alterations that occurred during chilling storage were examined in this study. This research’s findings can be used in the manufacture of chicken products and the design of the corresponding process, including the cooking and pre-cooking steps. Furthermore, understanding the volatile changes that occur during chilling can help design storage settings to limit product alterations.
Materials and Methods
Chicken breast meat (Ross308 strain, 42–45 d of age, storage at 5°C–8°C; 9.04% protein, 0.74% fat, and 0.57% carbohydrate) was purchased from the local market. It was diced into cubes measuring 1 cm×1 cm×1 cm and then boiled in water with pH adjustments of 6.0, 7.0, 8.0, and 9.0 made using sodium bicarbonate and citric acid until the core temperature of the chicken reached 95°C for 5 min. The ratio of chicken and water was 1:10. The samples were divided into two parts: 1) to evaluate the volatile compound immediately, and 2) to analyze the volatile compound after 7 d of storage at 4.0±1.0°C (chilling).
The headspace volatile compounds in the chicken were analyzed using the Thermo Scientific Trace 1300 gas chromatography-mass spectrometry (GC-MS; Thermo Fisher Scientific, Oxford, UK). The volatile compounds were collected and separated with a TG-5slims column with dimensions of 0.25 mm×30 mm×0.25 μm (Thermo Fisher Scientific) using helium (UHP, 99.999%) as a carrier gas at a constant flow rate of 1.6 mL/min. With the modified procedure of Bhadury et al. (2021), the initial oven temperature was held at 50°C for 2 min before ramping at 5°C/min until 125°C for 10 min, and then at 10°C/min until 200°C for 2 min. The total run time was 26.50 min. Eluted compounds that exited the GC column were separated by a splitter at a ratio of 2:3 before entry into the mass selective detector (MSD 5975, Agilent Technologies, Santa Clara, CA, USA). The MSD was operated in the electron impact mode at 70 eV with 35 to 500 scanning m/z. The identification of the separated compounds was performed by comparing the mass spectra to the NIST02 library available in the GC system. The percentage peak area method was used to present the content of each specified component by evaluating it as a proportion of the area of the target component to the total area of all of the detected peaks. Each treatment was examined using 10 g of boiling chicken cubes in triplicate. Every analyte was injected in triplicate.
Results and Discussion
The volatile compounds obtained at different pHs of the boiling water, in chicken breast meat were detected using GC-MS. In all, 21 volatile compounds were detected among eight samples (chicken boiled at pHs of 6.0, 7.0, 8.0, and 9.0 and those chilled for 7 d), which could be divided into seven categories. Among all of the volatile compounds were one amine, one ester, one furan, two ketones, eight alcohols, and eight aldehyde compounds. It was found that the pH of the boiling water not only affected the number of volatile compounds but also their relative contents. Moreover, changes were observed in both compound type and quantity during chilling storage. The highest concentration of volatile compounds detected in the chicken was that of amine, followed by that of aldehyde, ketone, alcohol, and acids, respectively, as shown in Fig. 1A. Most of these compounds (except amine) were derived from the Maillard reaction or the thermal degradation of lipids (Ruiz et al., 2002). Amine, alcohol, ketone, alcohol, and acid volatiles were found in chicken boiled at a pH of 6.0, but no acid was found in chicken boiled at pHs of 7.0, 8.0, and 9.0. The free OH− in the medium with pHs of 7.0, 8.0, and 9.0 most likely neutralized the free H+ in the medium; moreover, a number of acids were destroyed by heat during boiling. Furthermore, alcohol was not detected in chicken cooked at a pH of 7.0. The volatile compounds changed after 7 d of chilling, as indicated in Fig. 1B. The concentration of amine in the boiled chicken reduced; in contrast, that of aldehydes and alcohols increased. Acid was only found in the chicken boiled at a pH of 6.0 as 0.2% and increased after storage for 7 d as 0.4%. In addition, ester (n-caproic acid vinyl ester) was not found in the chicken boiled at any pH, but it was detected in the chicken boiled at any pH studied after 7 d of chilling storage. It was probably produced from the esterification of the existing alcohol and carboxylic acid (Hwang et al., 2020). In addition, 2-pentyl furan, a non-carboxyl compound derived from linoleic acid oxidation (Wall et al., 2019), lipid oxidation (Zhao et al., 2022), and the Maillard reaction (Niu et al., 2016), was identified to be responsible for fatty and cooked-meaty aromas (Niu et al., 2016; Zhao et al., 2022), which had a very low aroma threshold (Wang et al., 2020a).
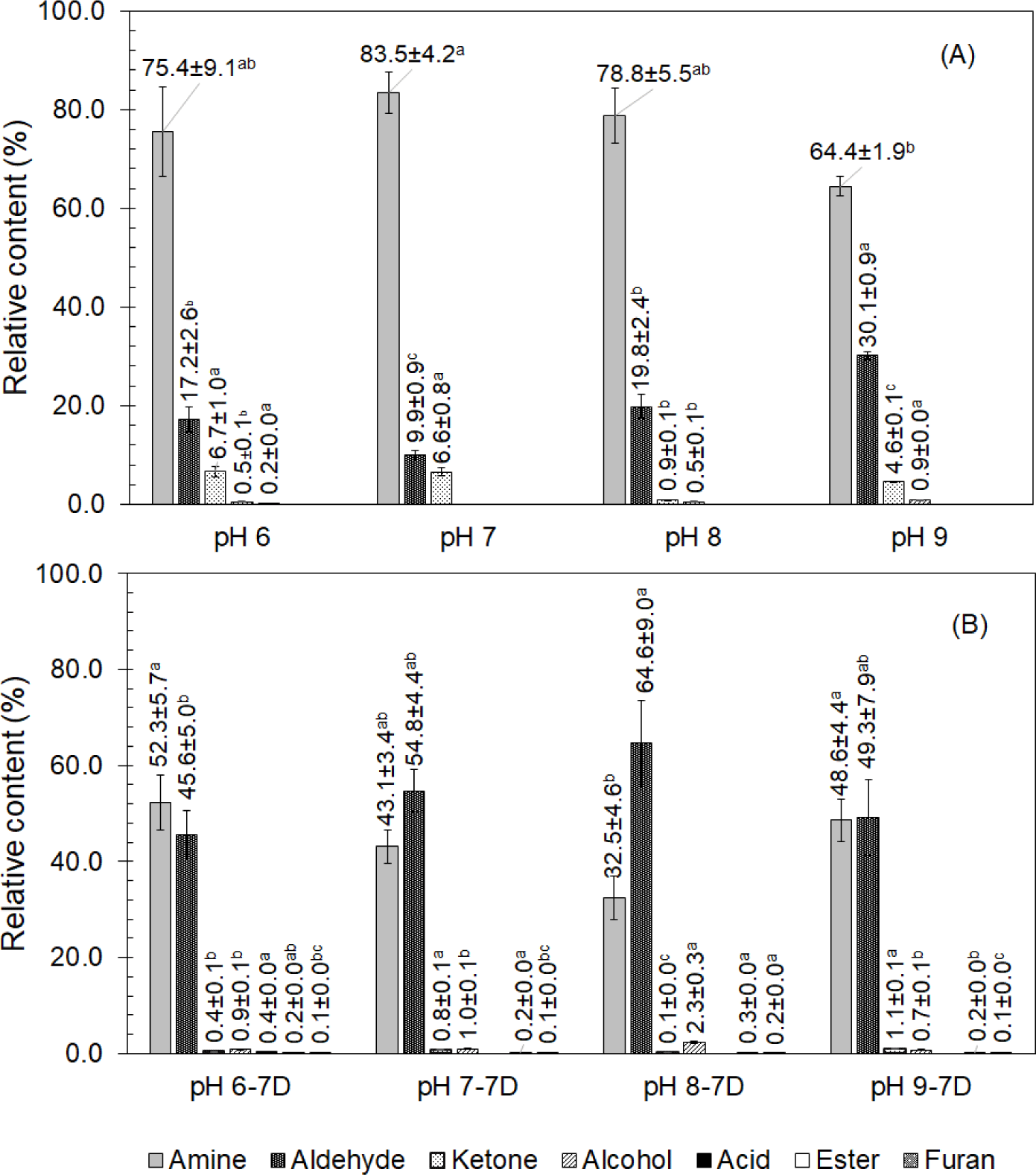
The only amine found in the chicken after boiling and chilling was (2-aziridinylethyl) amine with a fishy flavor (Xu et al., 2019). As it contained nitrogen, it was possibly related to the amino acid degradation during boiling. A reduction in its relative content was found after storage at a chilling temperature (lower than 10°C), but the relative contents of the other products, particularly the aldehydes, increased. The volatile compounds from chicken boiled at a pH of 9.0 included the least amount of (2-aziridinylethyl) amine and the most aldehydes; in contrast, those boiled at a pH of 7.0 exhibited an increase in the formation of (2-aziridinylethyl) amine while inhibiting the aldehyde release. However, after 7 d of chilling, the amount of (2-aziridinylethyl) amine in all of the samples (chicken boiled at each pH tested) decreased while that of the aldehydes increased. The aldehyde content in the chicken boiled at pHs of 7.0, 8.0, and 9.0 became not significantly different (p<0.05) after chilling for 7 d. It was indicated that the boiling medium pH of 7.0 inhibited the aldehyde production in the fresh boiling but enhanced the generation during storage up to 5.5 times (from 9.9% to 54.6%). This change altered the aroma in the chicken from fishy to aldehyde-type. The changes in type and contents of volatiles during chilling storage were because the pH condition of the boiling process affected the oxidation rate of the volatiles (Hassanzadeh et al., 2022a; Hassanzadeh et al., 2022b), fatty acids (Ba et al., 2013), and lipids (Kim et al., 2016).
Prior research (Jung et al., 2023; Petrucci et al., 2016) indicates that heating provided molecular energy to break atom-to-atom bonds, resulting in smaller molecules or volatiles, but it also led to volatile degradation (Yuan et al., 2023). Moreover, Yang et al. (2017a) found that the pH of the solution affected the volatility of the compounds through two processes: 1) decomposition, in which a low or high pH caused some compounds to break down into smaller, more volatile molecules, and 2) acid and base reactions, which result in charged ions, which are less volatile than neutral molecules. This investigation included the effects of dissolving the molecules, releasing the volatile molecules through heat, and enhancing the volatile stability under pH conditions.
Aldehydes were the second-most-abundant volatile compounds in the chicken, both after boiling and chilling. They significantly contributed to the overall aroma of cooked chicken because of their low threshold values (Ba et al., 2010). As shown in Fig. 2, after boiling, five aldehydes, namely hexanal, pentanal, nonanal, heptanal, and octanal, were detected in the chicken boiled at pHs of 6.0, 8.0, and 9.0, but three aldehydes, namely hexanal, pentanal, and nonanal, were detected in the chicken boiled at pH 7.0. Among them, nonanal and heptanal had pleasant meat flavors, and octanal had a sweet orange flavor. Benzaldehyde, an organic compound formed by replacing a hydrogen of benzene with an aldehyde group, contributed an almond smell (Zhao et al., 2021).
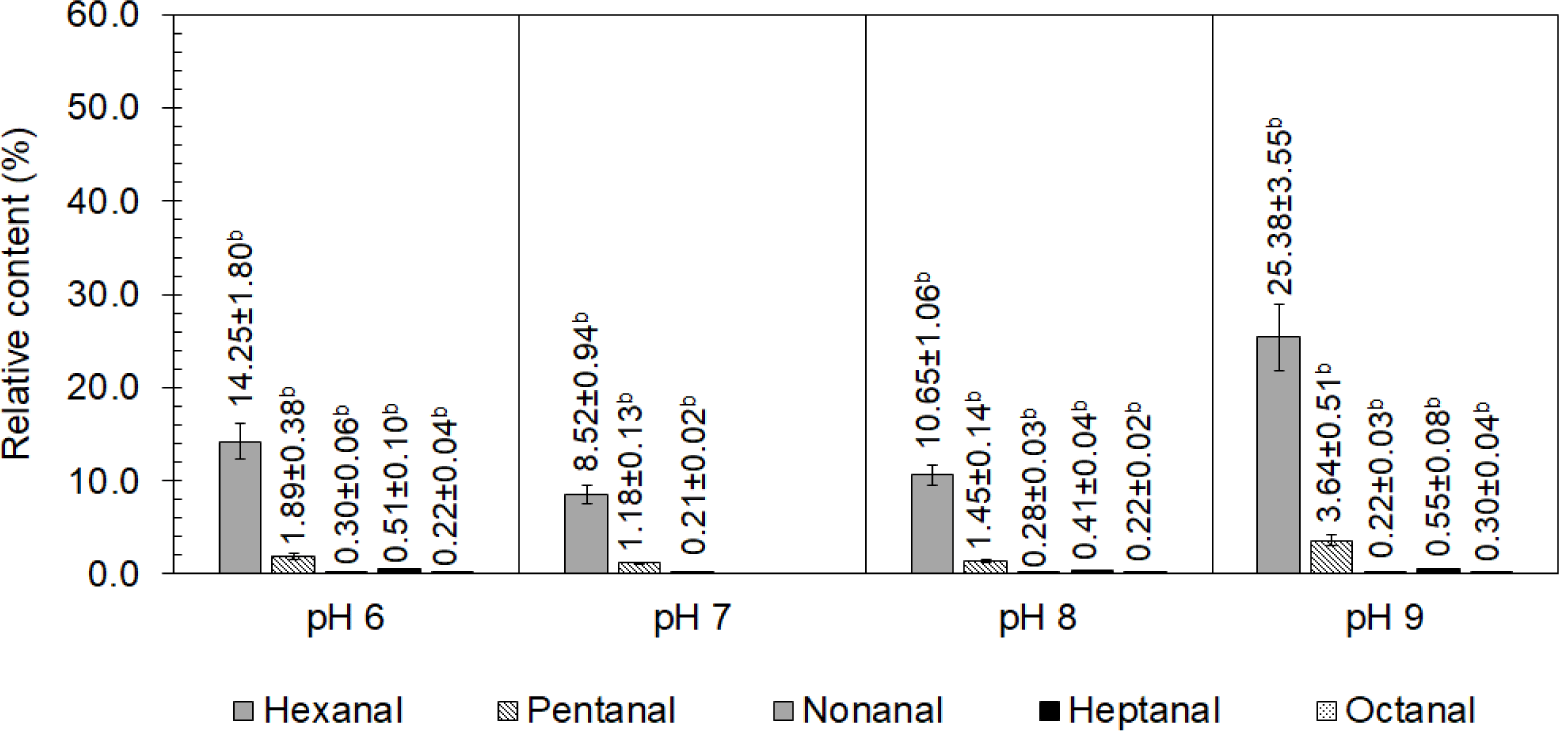
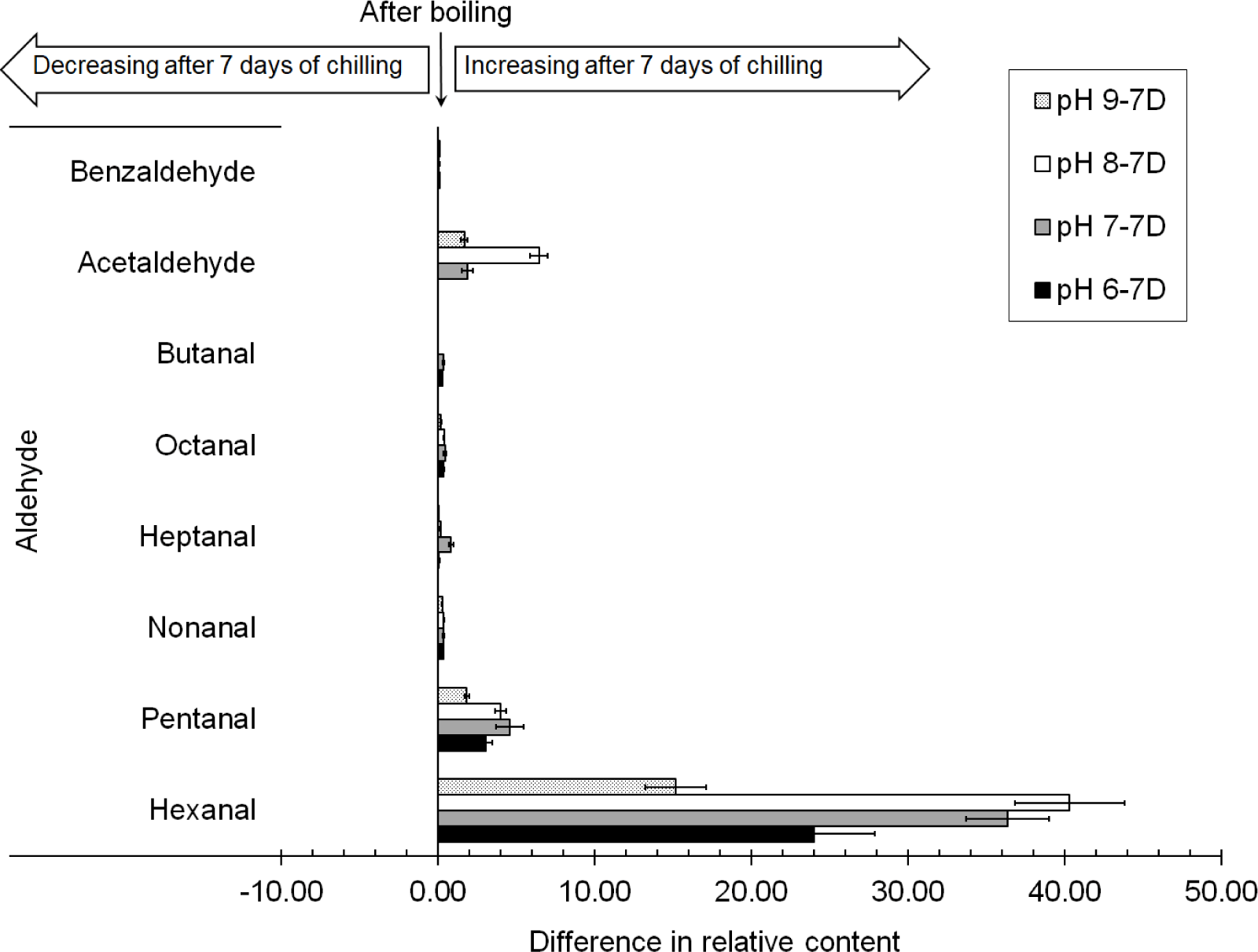
However, more types of aldehydes were found after chilling for 7 d. Butanal was detected in the chicken boiled at a pH of 6.0 after chilling for 7 d, while acetaldehyde and benzaldehyde were found in the chicken boiled at pHs of 7.0, 8.0, and 9.0 and chilled for 7 d. It was found that acetaldehyde formed in a high concentration in the chicken boiled at a pH of 8.0 and chilled for 7 d as the 6.42% relative content increased, as shown in Fig. 3; it caused a pungent odor, which was unpleasant, in the chicken (Luttrell, 2009). A small amount of benzaldehyde (~0.06% relative content increase) was detected in the chilled chicken, which was boiled at pHs of 7.0, 8.0, and 9.0; however, its high threshold (Acree and Arn, 2004) presented no effect on the sensory reception. The relative contents of hexanal and pentanal clearly increased in the chicken boiled at every pH of water after chilling for 7 d. The highest increase in the relative concentration of hexanal after 7 d of chilling in the chicken boiled at a pH of 8.0 (40.29%), followed by that boiled at pHs of 7.0 (36.34%), 6.0 (23.99%), and 9.0 (15.17%), respectively. Aldehydes were identified as the primary odor components in cooked chicken because of their high volatility and low threshold (Shi et al., 2019). Previous research (Yang et al., 2017b) has demonstrated that aldehydes are mostly produced via lipid oxidation. Hexanal and heptanal, known for their unpleasant rancidity and green odor (Kobayashi et al., 2016; Li et al., 2020), are mostly formed by the oxidation of linoleic acid and arachidonic acid, whereas octanal and nonanal are formed through the oxidation of oleic acid (Watanabe et al., 2015), which is high in chicken meat (Kim et al., 2020). Moreover, pentanal, nonanal, and octanal have been reported to be formed from the oxidation of unsaturated fatty acids and the thermal oxidative decomposition of fat (Sampaio et al., 2012).
According to Fig. 4, alcohol was not produced in the chicken boiled at a pH of 7.0, but two alcohols, namely 1-pentanol and 1-octen-3-ol, were found in the chicken boiled at a pH of 6.0. Both 1-pentanol and 1-octen-3-ol are the oxidation products of fatty acids composed of linoleic acid (Pratt et al., 2011) and arachidonic acid (Jerković et al., 2021), respectively. 1-Pentanol and 2-hexyl-1-octanol were also found in the chicken boiled at pHs of 8.0 and 9.0, while 1-octen-3-ol, a product of the auto-oxidation of linoleic acid that supplied a mushroom aroma (Feng et al., 2019) and green flavor (Zhang et al., 2023), was detected in the chicken boiled at a pH of 9.0 but not at a pH of 8.0. Because alcohols are the main products of fat oxidation and decomposition and generate the aroma volatiles (Mikš-Krajnik et al., 2016), the difference in the type and concentration of the detected volatile compounds in the boiled chicken meat was attributed to the differences in the fat decomposition at different pHs.
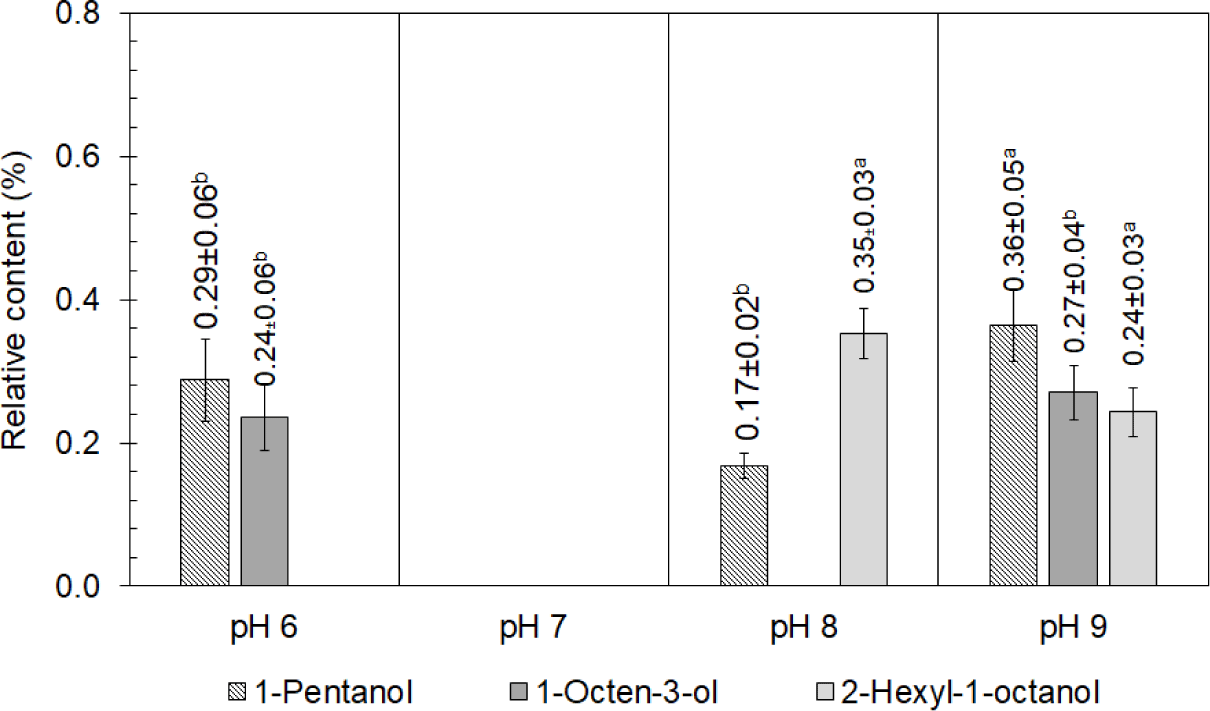
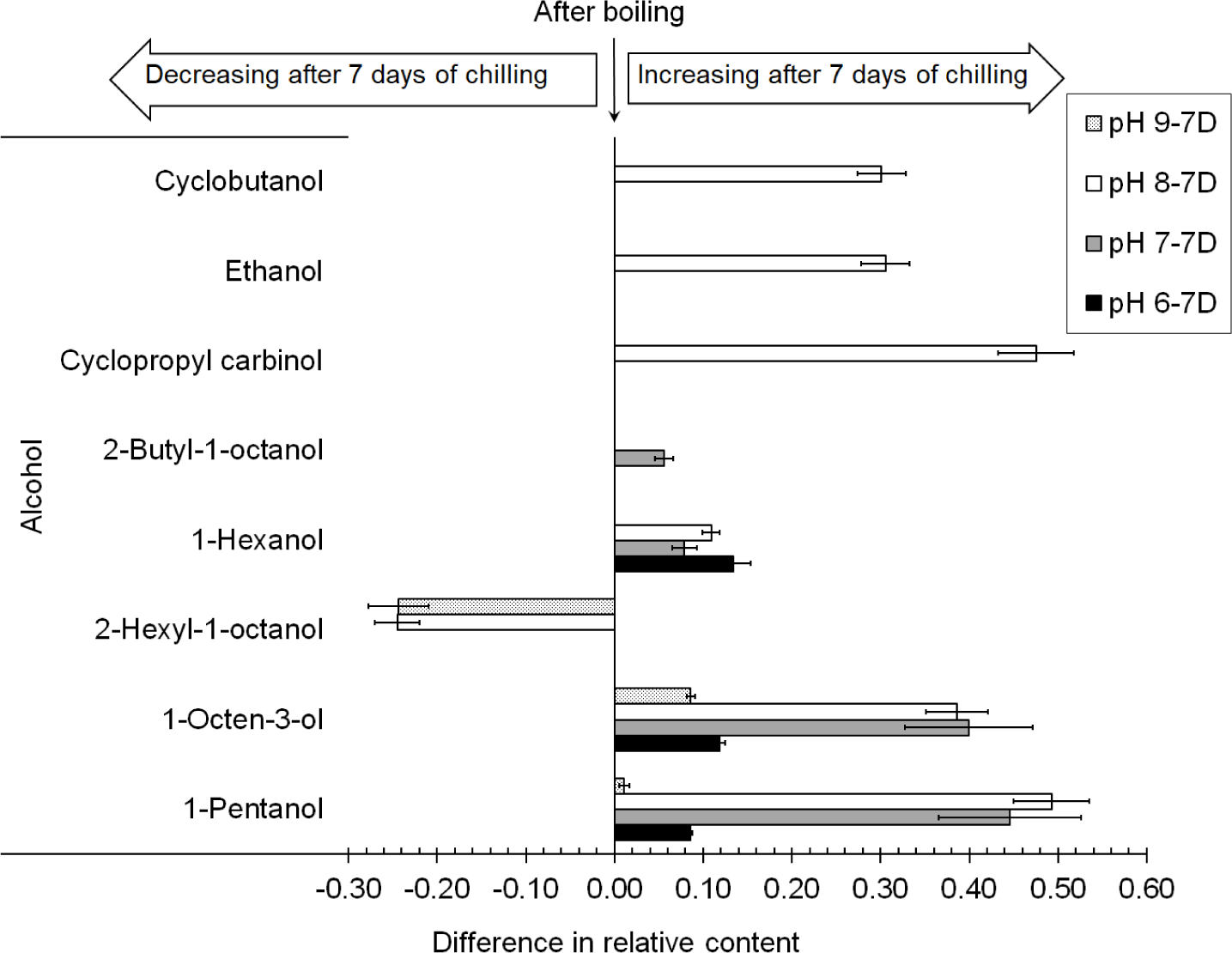
After storage under chilling conditions for 7 d, 1-pentanol and 1-octen-3-ol detected in the chicken boiled at all of the studied pHs increased, while 2-hexyl-1-octanol detected in the meat boiled at pHs of 8.0 and 9.0 decreased by a relative concentration of 0.24, as shown in Fig. 5. It was possible that 2-hexyl-1-octanol was a substance for some reactions or a substrate for bacterial growth during the chilling storage. The increases in 1-pentanol and 1-octen-3-ol in the chicken boiled at pHs of 8.0 and 9.0 were quite similar, with relative concentrations of around 0.45–0.49 and 0.39–0.40, respectively. As both 1-pentanol and 1-octen-3-ol were formed during the oxidation of the fatty acids, the results indicated that there was still fatty acid oxidation during storage, particularly in the chicken boiled at pHs of 7.0 and 8.0. A new volatile compound, 1-hexanol, contributing an oily odor (Niu et al., 2016), was detected in the chicken boiled at pHs of 6.0, 7.0, and 8.0. Even if alcohols were not found in the chicken after boiling at a pH of 7.0, as shown in Fig. 4, four alcohols, namely 1-pentanol, 1-octen-3-ol, 1-hexanol, and 2-butyl-1-octanol, were detected after chilling for 7 d, as shown in Fig. 5. Moreover, various alcohols were present in the chicken boiled at a pH of 8.0 after chilling for 7 d: 1-octen-3-ol, 1-hexanol, 2-hexyl-1-octanol, cyclopropyl carbinol, ethanol, and cyclobutanol. Most of them, such as 1-pentanol, 1-hexanol, and 2-hexyl-1-octanol, gave off fatty odors. Ethanol, which was detected only in the chicken boiled at a pH of 8.0 and chilled for 7 d with a relative content of 0.30±0.03%, indicated the spoilage of the boiled chicken (Klein et al., 2018; Mikš-Krajnik et al., 2016). This implied that the chicken boiled at pHs of 6.0, 7.0, and 9.0 had a longer shelf life for chilling storage.
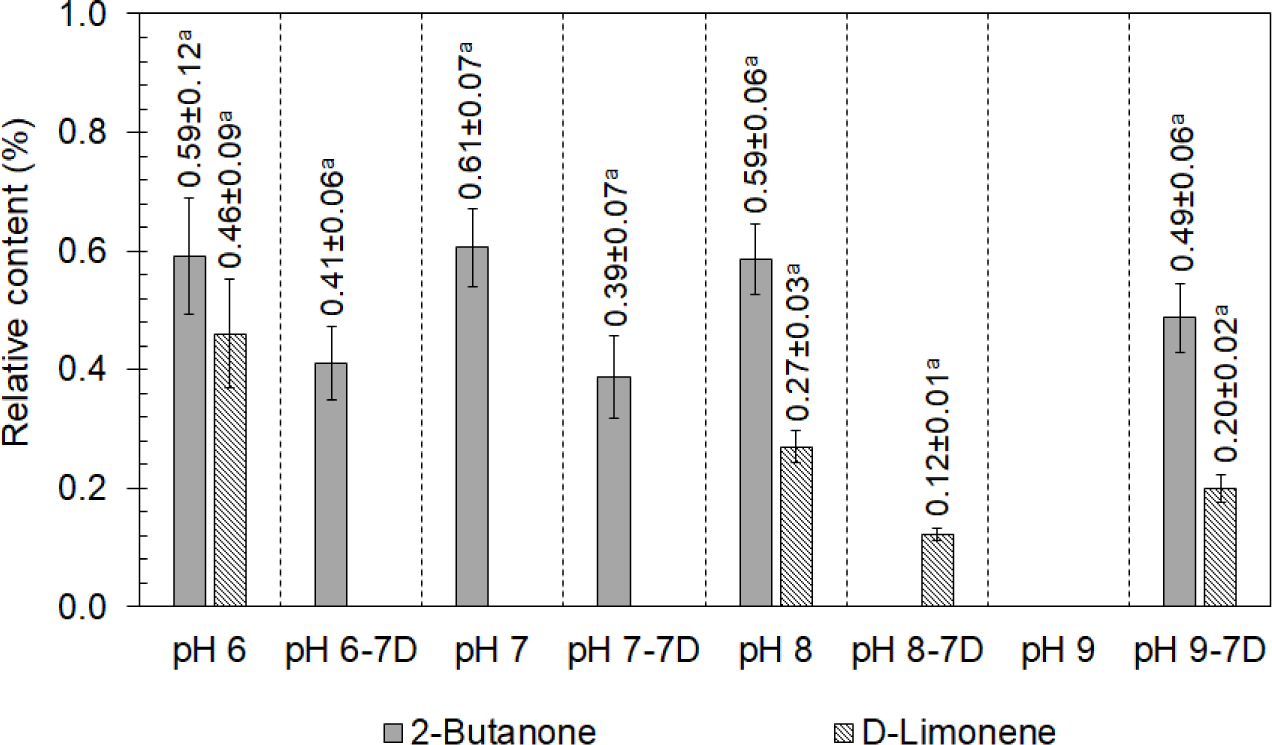
2-Butanone compounds, having a pungent odor (Zhao et al., 2021), were detected in the chicken boiled at pHs of 6.0, 7.0, and 8.0, respectively, as shown in Fig. 6. D-limonene, one of the most common terpenes in nature found in beef (Cañedo et al., 2009) and chicken (Ramaswamy and Richards, 1982), which was probably from the feed (Bampidis et al., 2005), was found in the chicken boiled at pHs of 6.0 and 8.0. No ketone was found in the chicken after boiling at a pH of 9.0, but both 2-butanone and D-limonene were detected after chilling for 7 d. In contrast, D-limonene found in the chicken after boiling at a pH of 6.0 disappeared after 7 d of chilling due to its low zeta potential at low temperatures in the chilling condition (Li and Lu, 2016). Moreover, it was found that the 2-butanone volatile was reduced in the chicken boiled at pHs of 6.0, 7.0, and 8.0 after chilling for 7 d. It was implied that the pungent odor from 2-butanone reduced during chilling, similar to the citrus odor from D-limonene, which also reduced after chilling.
Conclusion
The volatile development and stability of boiled chicken were investigated using a headspace GC-MS after boiling and during the chilling storage period. The findings revealed that boiling chicken flesh at various boiling medium pH levels affected the generation of volatiles and alterations that occurred after storage. Amines, aldehydes, alcohols, ketones, acids, esters, and furans all responded significantly to the pH of the boiling medium and chilling storage (7 days). The maximum concentration of amine [(2-Aziridinylethyl) amine, boiled at pHs of 6.0–8.0] was found after boiling. In contrast to the alterations observed after 7 days of chilling, amines showed the greatest decline, while aldehydes showed the greatest increase. In specifics, after the chicken was chilled for 7 days, it was found to include n-caproic acid vinyl ester (ester), 2-pentyl furan, butanal, acetaldehyde, benzaldehyde, cyclopropyl carbinol, ethanol, and cyclobutanol, depending on the pH of the boiling medium. This study showed that heat in the boiling medium with varying pH levels changed the production of distinct volatiles in the boiled chicken meat. The primary causes of the volatiles' altered composition during chilling were Maillard and oxidation reactions. Various types of volatiles in chicken were generated after boiling it in a medium with a low pH (a pH of 6.0), but the off-flavors (aldehydes) created after chilling were found mostly in the chicken meat boiled at a high pH (pHs of 8.0 and 9.0). The pH of the boiling medium should be taken into account in order to improve the changes in volatiles or flavor in cooked chicken during chilling storage.













