Introduction
The demand for chicken meat has led to a year-over-year increase in chicken production (Sujiwo and Ariyadi, 2023). Prized for its affordability and well-balanced nutrient profile, chicken has become a cornerstone protein source in global diets. Rich in protein, low in saturated fat, and replete with essential vitamins and minerals, chicken offers substantial nutritional value (Donma and Orkide, 2017; Jilo and Hasan, 2022; Kralik et al., 2018). This popularity is expected to continue, with projections indicating a significant rise in poultry consumption, including chicken, by 2030 (van der Laan et al., 2024). Global consumption is estimated to exceed 120 million tons, with per capita consumption reaching an anticipated 17 kilograms annually (Reay, 2019). While broilers currently dominate the global chicken meat market, a resurgent professional and consumer interest is emerging in native breeds. The growing interest in native chicken breeds stems from flavor profiles, genetic richness, adaptability to local environments, potential contributions to sustainable agriculture, and cultural heritage preservation (Barido et al., 2022; Gao et al., 2023; Yuan et al., 2022). In line with the global trend, the popularity of native chicken breeds has also seen a resurgence in South Korea. The growing domestic demand for Korean native chicken (KNC) presents a significant opportunity for revenue generation (Barido et al., 2022).
Native chicken breeds, such as KNC, are not only prized for their unique flavor and superior nutritional value, but also hold deep cultural significance in many regions, including South Korea. These breeds are often linked to traditional farming practices and regional cuisines, playing a vital role in local economies. The preservation and commercialization of KNC, therefore, contributes not only to agricultural biodiversity and food security but also to the economic empowerment of rural communities. By supporting native breed production, the poultry industry can tap into the growing consumer interest in specialty products, offering a premium alternative to conventional broilers (Park et al., 2022).
In response to the recognition of the valuable genetic traits and desirable taste profile of the KNC, the Korean government initiated a program to preserve and facilitate the commercialization of this native chicken breed (Jin et al., 2017). Consequently, a number of breeds were developed, including Woorimatdag No. 1 (WRMD1) as well as Woorimatdag No. 2 (WRMD2). The objective of developing WRMD1 was to create a more affordable product while maintaining its flavor characteristics. Studies have demonstrated that WRMD1 contains higher levels of both taste-active and bioactive compounds than commercially available broilers (Jayasena et al., 2015). However, its slower growth rate has presented a challenge in meeting consumer demand. To address this issue, WRMD2 was developed by crossbreeding KNC with meat-type breeds, resulting in improved growth rates while maintaining desirable meat quality (Choi et al., 2015).
Freezing is an effective method for preserving the quality of chicken meat by preventing microbial growth and the activity of enzymes, consequently prolonging its shelf life. This technique allows for the long-term storage and transportation of poultry products, thereby ensuring their availability beyond the limits of seasonal production cycles. Although freezing is a well-established method for maintaining the integrity of meat proteins, it inevitably results in a decline in quality compared to fresh meat (Leygonie et al., 2012). The formation of ice crystals during the freezing process disrupts proteins and causes the migration of intracellular water to the extracellular space, resulting in a dry texture, tougher consistency, and protein degradation (Leygonie et al., 2012). Nevertheless, the selection of freezing and thawing techniques can markedly attenuate the detrimental consequences of meat freezing. A quick-freezing method facilitates the development of smaller ice crystals within cells and tissues, which reduces physical damage and deterioration in meat quality (Yun et al., 2021). The way meat is thawed is also of great consequence with respect to the maintenance of meat quality. The use of slow thawing methods, such as refrigeration, room temperature, or cold water, has been shown to result in a loss of quality due to prolonged exposure to temperatures within the bacterial danger zone and uneven thawing (Chandirasekaran and Thulasi, 2010). While accelerated techniques, including high-pressure, ohmic, and microwave thawing enhance both the speed and quality of the thawing process, their application is still constrained by certain limitations (Arshad et al., 2023).
The effects of freeze-thawing on the chemical and physical qualities of broiler meat have been the subject of extensive research. Studies have investigated aspects such as protein structural changes, lipid oxidation, and alterations in water-holding capacity (WHC) along with textural changes (Arshad et al., 2023; Jemziya and Rifath, 2022; Pereira et al., 2022; Shin et al., 2023; Villegas-Cayllahua et al., 2023). The existing literature on the effects of freezing and thawing on meat quality lacks sufficient research on the impact of these processes on KNC breeds, which possess distinct genetic and organoleptic characteristics (Jin et al., 2017). While previous studies have examined the physical, chemical, and flavor properties of KNC thigh meat using standard freezing and thawing techniques (Barido et al., 2022; Jung et al., 2015), this study aims to address the existing gap in the literature by investigating the physicochemical, microbiological, sensory, and flavor attributes of KNC breast meat using a range of freezing and thawing methods. Such knowledge is essential for maintaining the nutritional and sensory qualities of the meat, as well as for supporting the commercial viability and preserving the cultural significance of these indigenous breeds. Thus, the objective of the study is to assess the physicochemical characteristics, sensory, and flavor-related properties of breast meat from two KNC breeds, WRMD1 and WRMD2, to those of broilers, under fresh and various freeze-thaw treatments.
Materials and Methods
A total of ten samples of each breed were obtained at a local market: the broiler group (n=10), the WRMD1 group (n=10), and the WRMD2 group (n=10). Upon receipt of the samples, they were immediately refrigerated at 4°C and transported to the laboratory under controlled temperature conditions. Upon arrival at the laboratory, the breast meat from each bird was meticulously dissected for further analysis.
Samples were subjected to slow freezing at –20°C (SF) then stored at this temperature and quick freezing at –70°C for 24 h (QF), followed by storage at –20°C. The frozen samples were stored for two months before the thawing process. Thawing methods included refrigerator thawing at 4°C for 8–9 h (RT), ambient temperature thawing at 22°C for 5 h (AT), water thawing at 15°C running tap water for 25–30 min (WT), and microwave thawing at 700 W for 4.5–6 min (MT). Fresh meat served as a control for quality and taste comparisons across all freezing and thawing conditions.
The proximate composition was analyzed in accordance with the methods delineated by the Association of Official Agricultural Chemists (AOAC, 1995). The moisture content was ascertained by subjecting the samples to drying in an oven at 105°C for 12 h. The Kjeldahl method was employed for the analysis of crude protein, while the determination of crude fat was conducted through ether extraction. The quantification of crude ash was carried out by incinerating the samples at 550°C.
The drip loss was calculated as the percentage ratio of the initial sample weight prior to freezing to the final weight of the sample following thawing. The drip loss value was calculated according to the following formula:
Cooking loss was determined by calculating the percentage difference in weight between the initial and final states, with each sample heated by placement in a vacuum-sealed bag submerged in water at 80°C for 30 min.
The cooking loss percentage was determined by the following calculation:
The pH was measured by first preparing a homogenate, which entailed blending 10 grams of the sample with 90 mL of distillate water for 30 consecutive seconds using a Polytron PT-2500 E homogenizer (Kinematica, Malter, Switzerland). Subsequently, the pH of the resulting homogenate was evaluated with the use of an Orion 230 A pH meter (Thermo Fisher Scientific, Waltham, MA, USA).
WHC was assessed using the method previously described (Jung et al., 2022). In brief, a 0.5 g portion of the sample, devoid of connective tissue, was subjected to a 20 min heating process at 80°C in a water bath, after which it was cooled at room temperature for an additional 10 min. Subsequently, the sample was subjected to centrifugal separation at 2,000×g for 20 min at 4°C to assess water loss. The WHC was determined by calculating the percentage of water loss in relation to the total moisture content of the sample.
Shear force measurements were conducted with a TA1 texture analysis system (Lloyd Instruments, Berwyn, IL, USA) equipped specifically with a V blade. The chicken breast samples were sealed in polyethylene bags and subjected to a 45 min thermal treatment at 75°C in a water bath. Thereafter, sections measuring 1×3×2 cm were excised and analyzed using a texture analyzer equipped with a 500 N loading cell and a crosshead velocity of 50 mm/min.
The color attributes of the meat, specifically CIE L*, CIE a*, and CIE b*, have been evaluated utilizing a Chroma Meter CR-400 (Minolta, Osaka, Japan). Prior to analysis, the device was calibrated using a reference plate of known white color (Y=93.60, x=0.3134, y=0.3194).
The total aerobic bacteria (TAB), total coliforms, and Escherichia coli counts were evaluated using Petrifilm counting plates, manufactured by the 3M Company (St. Paul, MN, USA). A total of 3 g of the samples were homogenized with 27 mL of sterilized saline solution using a mechanical stomacher (BagMixer 400, Interscience, Saint-Nom la Bretèche, France). A 1-mL aliquot of each homogenate was inoculated onto Petrifilm plates and subjected to incubation at 37°C for a period of 48 h. Colony counting was performed and the results were presented as Log CFU/gram.
A sensory analysis was performed with the participation of a panel of 15 college students, aged between 21 and 38 years. The chicken breast samples were heated in a water bath to a final core temperature of 70±2°C and then cut into pieces measuring 1×1×3 cm. The panelists evaluated the samples on a scale of 1 to 9 for various attributes, including appearance, aroma (rated on a scale of 1 to 9, with 1 indicating a very poor rating and 9 indicating a very good rating), off-flavor (rated on a scale of 1 to 9, with 1 indicating a very strong off-flavor and 9 indicating a very weak off-flavor), tenderness (rated on a scale of 1 to 9, with 1 indicating a very tough sample and 9 indicating a very tender sample), juiciness (rated on a scale of 1 to 9, with 1 indicating a very dry sample and 9 indicating a very juicy sample), and overall acceptability (rated on a scale of 1 to 9, with 1 indicating a very unacceptable sample and 9 indicating a very acceptable sample). This study was conducted in accordance with the ethical standards of the Institutional Review Board at Kangwon National University (KWNUIRB-2021-05-004-001).
Fatty acid composition was analyzed following the method of Kim et al. (2020). A 2 g sample was homogenized in a Folch solvent mixture (chloroform:methanol=2:1) with the addition of 40 μL butylated hydroxyanisole. The homogenate was filtered through Whatman No. 1 paper, and the lipid phase was isolated by mixing the filtrate with 4 mL of 0.88% potassium chloride, followed by centrifugation at 783×g force for 10 min. The lipid layer was concentrated under nitrogen gas. Fatty acid methyl esters (FAMEs) were prepared by saponifying 25 mg of the lipid extract with 1.5 mL of 0.5 N sodium hydroxide in methanol at 100°C for 5 min. After adding 1 mL of 10% boron trifluoride (BF3) and heating at 100°C for 2 min, the mixture was treated with 2 mL of isooctane and 1 mL of saturated sodium chloride solution, followed by centrifugation at 783×g for 3 min. The top layer of isooctane containing the FAMEs was extracted and analyzed using an Agilent 7890N gas chromatography system (Omegawax 250 column, Agilent, Santa Clara, CA, USA). Helium was used as the carrier gas at a flow rate of 1.2 mL/min with a split ratio of 1:100. Fatty acids were identified by comparing retention times with a commercial standard mixture (PUFA No. 2-Animal Source; Supelco, Bellefonte, PA, USA).
Nucleotide-related compounds were quantified following the method of Barido et al. (2022) with modifications. A 5 g minced sample was homogenized in 25 mL of 0.7 M perchloric acid (PCA). The homogenate was centrifuged at 2,000×g for 15 min at 0°C and filtered. The extraction was repeated with an additional 20 mL of 0.7 M PCA. The combined filtrates were neutralized to pH 6.5 with 5 N potassium hydroxide and adjusted to 100 mL with PCA. After cooling and centrifugation, the solution was filtered through a 0.22 μm syringe filter. Nucleotide analysis was performed using an Agilent 1260 Infinity HPLC with a Nova-Pak C18 column. Detection occurred at 254 nm, with the mobile phase composed of 1% trimethylamine phosphoric acid (pH 6.5). The quantification was based on standard curve created from hypoxanthine, inosine, inosine monophosphate (IMP), adenosine monophosphate (AMP), adenosine diphosphate (ADP), and adenosine triphosphate (ATP) reference standards (Sigma Aldrich, St. Louis, MO, USA).
Volatile organic compounds (VOCs) were analyzed using headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry (GC-MS), following Lv et al. (2019). A 5 g meat sample was homogenized in a 20 mL glass vial and incubated at 60°C for 25 min. A DVB/CAR/PDMS fiber (Sigma Aldrich) was exposed to the headspace at 60°C for 30 min to absorb VOCs. The fiber was conditioned at 250°C for 30 min before analysis. GC-MS analysis was performed using an Agilent 8890 gas chromatograph and Agilent 5977 B mass spectrometer. The VOCs were separated on a DB-5MS capillary column (30 m×0.25 mm, 0.25 μm film; Agilent). Helium was used as the carrier gas at 1.3 mL/min in splitless mode. The oven temperature started at 40°C for 5 min, then increased by 5°C per min until reaching 250°C, where it was held for 5 min. The mass spectrometry conditions were set with electron impact ionization at 70 eV, scanning from 30 to 300 Dalton (amu). Compound identification was based on retention indices relative to n-alkanes (C8-C24) and compared to mass spectral libraries (NIST 20). Results were represented as area units (A.U.) multiplied by 10°C (Supplementary Table S1). Flavor attributes of the identified compounds were characterized using databases such as Flavornet, FooDB, and PubChem (Barido et al., 2022; Sujiwo et al., 2024).
The experiments described were replicated five times. A one-way analysis of variance (ANOVA) with the General Linear Model procedure was conducted using SAS statistical software (Release 9.4; SAS Institute, Cary, NC, USA) to perform the statistical analysis. The Tukey method was used to test the significance between treatment means at the 5% level. The data was presented as means and SDs. Partial least squares discriminant analyses (PLS-DAs) were conducted using the online software package MetaboAnalyst 5.0 to generate the heatmap (Fig. 1; Man et al., 2023).
Results and Discussion
Table 1 presents the proximate composition of broiler and KNC breast meat, highlighting differences influenced by both breed and freeze-thaw methods. Overall, the moisture content of the broilers was significantly higher than that of the KNC after the freeze-thaw treatment. Native chicken breeds and broilers have different genetic backgrounds, which can influence muscle composition and structure. These genetic factors can affect the WHC and moisture content of the meat (Mussa et al., 2022). The moisture content of the broilers was comparable to that of fresh meat with the SF-AT and SF-WT treatments. Furthermore, quick freezing with any thawing method preserves moisture at a level comparable to that of fresh meat. For WRMD1, only QF-WT maintained moisture content comparable to that of fresh meat. For WRMD2, no significant differences in moisture content were found between fresh and frozen-thawed samples. The moisture levels of chicken breasts in this study ranged from 74.53% to 76.60%, which is consistent with previous findings by Oliveira et al. (2015). The lower moisture content in some frozen-thawed samples may be attributed to the freezing and thawing treatments. As Jeong et al. (2011) noted, freezing, preservation, and defrosting can remove water and other components from meat, affecting its texture and taste. The freeze-thaw process also impacted on the crude protein and crude lipid content, except for WRMD1. As Jeong et al. (2011) showed, this process causes muscle protein denaturation and lipid oxidation. From a breed perspective, certain frozen-thawed samples showed that crude protein content was higher in WRMD2 compared to broilers. In contrast, crude lipid and crude ash content varied, with SF-RT showing higher crude lipid content in broilers, while fresh, SF-WT, and QF-WT samples had higher crude ash content in broilers compared to native chickens. This is consistent with Choe et al. (2010), who found that the macronutrient levels of meat were strongly affected by breed, with the macronutrient levels of native chickens generally higher than those of broilers.
Table 2 shows the physicochemical characteristics of broiler breast meat and KNC breast meat subjected to different freeze-thawing methods. Breed and freeze-thawing methods significantly influenced drip loss, except for SF-WT and QF-MT samples, which showed no breed-related differences. Drip loss varied from 1.6% to 8.05%, exceeding previous reports by Frelka et al. (2019) and Oliveira et al. (2015). QF-RT was the most effective method for preserving water content across all breeds, as evidenced by the lowest drip loss. This aligns with the established practice of rapid freezing and low-temperature thawing. Rapid freezing minimizes cellular damage and preserves the integrity of muscle fibers by forming small ice crystals, reducing drip loss, and enhancing meat quality (Biglia et al., 2022). Native chicken meat exhibits lower drip loss than commercial broiler chickens, likely due to its denser muscle structure and slower growth rate, which contribute to better connective tissue and enhanced moisture retention (Ali et al., 2021; Ismail and Joo, 2017).
Cooking loss was influenced by breed and freeze-thawing methods, except for QF-RT and QF-MT. Broiler SF-MT and QF-MT resulted in cooking loss similar to fresh meat. In WRMD2, SF treatments had similar cooking losses to fresh meat, while QF treatments had higher cooking loss. Interestingly, for the WRMD1 breed, cooking loss for all freeze-thaw treatments were not different from fresh meat (p>0.05). When comparing breeds, WRMD2 exhibited significantly lower cooking loss than the broiler in the SF-RT, SF-AT, and QF-WT treatments. The cooking loss values in this study ranged from 20.18% to 26.49%, which is consistent with previously published data (Villegas-Cayllahua et al., 2023).
The pH values of the freeze-thaw treatments for all breeds were not significantly different from the fresh samples. The pH ranged from 5.48 to 6.01, which is within the standard range found in earlier studies (Ali et al., 2021; Bai et al., 2023). A notable finding was observed in WRMD1, which exhibited the lowest pH value compared to broilers and WRMD2 (p<0.05), except for the SF-AT treatment. Meat pH is influenced by various factors, including genetics, age, and post-mortem handling. The WRMD1 lower pH may be due to native chicken behavior. Their potentially more aggressive behavior can lead to elevated stress levels, increased glycogen utilization, subsequent lactic acid buildup, and ultimately, a lower post-mortem pH (Ali et al., 2021). Additionally, native chickens have lower growth performance, resulting in an older slaughter age. Older birds tend to have lower pH levels in breast meat (Glamoclija et al., 2015).
The WHC was largely unaffected by freeze-thaw treatments and breed, except for QF-RT where WRMD2 had lower WHC than broilers and WRMD1. The WHC values observed in this study fell within the normal range for chicken breast meat, consistent with previously published data (Frelka et al., 2019; Kim et al., 2020). Freeze-thaw treatments preserve WHC similarly to fresh meat, regardless of breed. The WHC is linked to post-mortem pH, which lower pH reduces WHC due to decreased net charge of myofibrillar proteins. Lactic acid accumulation from anaerobic glycolysis plays a significant role in postmortem pH decline (Barido et al., 2022). Rapid freezing helps preserve protein structures, thereby enhancing WHC and maintaining meat juiciness and quality (Biglia et al., 2022).
Shear force was unaffected by freeze-thaw treatments, remaining consistent with fresh meat, regardless of breed or freeze-thaw method. In terms of breed comparison, WRMD1 generally exhibited the highest shear force value among the breeds, indicating tougher meat. This was observed in the SF-RT, SF-AT, SF-WT, QF-AT, and QF-WT treatments. This can be attributed to the fact that WRMD1, being a purely native breed, tends to have higher muscle fiber density and smaller muscle fiber diameters compared to commercial breeds (Ali et al., 2021). Additionally, native breeds typically grow more slowly than commercial meat-type breeds. This slower growth allows for more extensive development of connective tissue and muscle fibers, resulting in a firmer texture (Jankowiak et al., 2023; Migdał et al., 2020).
Meat color is critical to sales as it signals freshness and is dependent on many factors including breed, age, and handling practices (Bae et al., 2014). Meat color is primarily determined by the postmortem myoglobin profile, a complex biochemical process that varies between different muscle types (Fletcher, 1999). In this study, meat CIE L* was partially affected by both breed and freeze-thawing treatments, except for WRMD1, where freeze-thawing treatments did not cause significant changes. For broiler meat, the CIE L* values for SF-RT, QF-RT, QF-WT, and QF-MT treatments remained consistent with fresh meat. While no significant differences in the CIE L* scores were observed between breeds for fresh meat, broiler meat had the lowest CIE L* scores after freeze-thaw treatments, except for QF-WT (p<0.05), compared to native chickens.
The CIE a* of WRMD1 was not affected by the freeze-thaw treatments (p>0.05). Broiler meat treated with quick freezing showed CIE a* values similar to fresh meat, except for QF-WT. While slow freezing significantly increased CIE a* in broiler meat compared to fresh sample (p<0.05). Native chickens had similar CIE a* to fresh meat, except for WRMD2 QF-AT. There were no significant differences in CIE a* between fresh and SF-AT samples, regardless of breed.
CIE b* was not significantly affected by freeze-thaw treatments for broiler and WRMD1 meat. For WRMD2, CIE b* was not significantly different (p>0.05) across freeze-thaw treatments. However, all quick freezing treatments resulted in significantly lower CIE b* values compared to fresh samples (Table 3).
Overall, while freeze-thaw treatments caused some changes in meat color, these effects varied among breeds and treatments. Meat color deterioration after freeze-thawing is due to myoglobin denaturation, reduced metmyoglobin reduction enzyme activity, increased myoglobin oxidation, and elevated levels of free radicals and pro-oxidants (Leygonie et al., 2012).
The microbiological condition of broiler and KNC breast meat under different freeze-thaw conditions is presented in Table 4. Compared to WRMD1 and WRMD2, broiler TAB was significantly lower, except for SF-AT and SF-MT. Freeze-thawing treatments had a minimal effect on broiler TAB. WRMD1 TAB was unaffected, except for QF-WT. WRMD2 TAB was higher after freeze-thawing. TAB values ranged from 2.38 to 4.90 Log CFU/g. The TAB values in this study ranged from 2.38 Log CFU/g to 4.90 Log CFU/g. Despite having higher TAB levels in native chicken compared to broiler, they are still within the acceptable limits for consumption according to the Korean Ministry of Food and Drug Safety (MFDS) guidelines, where the limit is set at 6.70 Log CFU/g (MFDS, 2018).
Broiler meat had the lowest coliform counts compared to native breeds. Broiler coliforms were detected in fresh and SF treatments. Native chicken coliforms were detected in all samples. WRMD1 and WRMD2 quick freeze treatments had lower coliforms than fresh samples. E. coli was detected only in the fresh samples of all breeds, as well as in the SF-AT and SF-MT treatments for broiler, and in the SF-AT and QF-WT treatments for WRMD2. In the other samples, E. coli was not detected. The E. coli counts in this study ranged from 0.08 to 1.28 Log CFU/g. Although detected, these levels are still considered safe for consumption according to MFDS regulations, which set the limit for E. coli in chicken meat at 4 Log CFU/g (MFDS, 2018).
Overall, freeze-thaw treatments did not significantly increase the microbial population in meat compared to fresh samples. This is in contrast to previous studies that reported higher total aerobic bacterial counts for frozen and thawed chicken meat than for fresh meat (Bae et al., 2014). Another study also indicated that chicken meat showed an increase in total bacterial count during thawing, especially with repeated freeze-thawing cycles (Mohammed et al., 2021).
From a breed perspective, broiler meat is considered to have the lowest microorganism population compared to native chicken. The higher bacterial counts observed in KNC compared to broiler meat can be attributed to several factors, primarily related to differences in processing environments and potential genetic factors. Commercial broilers are typically raised in highly controlled environments with strict hygiene protocols, which include optimized management practices during slaughtering, evisceration, and processing. These measures significantly reduce the risk of bacterial contamination (Julqarnain et al., 2022). In contrast, KNC are often processed in smaller, traditional, or artisanal settings where hygiene standards may not be as rigorously enforced. These environments may lack advanced equipment or have more manual handling, increasing the risk of contamination from the surroundings, equipment, or handlers (Rouger et al., 2017). Moreover, genetic factors may also be involved in the susceptibility of bacteria. A study reported that genetic factors associated with different breeds can influence microbial communities in chickens (Chen et al., 2023). These microbiotas live in different internal and external body locations, including feathers, skin, digestive tract, and lungs, improper slaughter controls can contaminate carcasses (Rouger et al., 2017). This suggests that breed differences could potentially affect the microbial condition of meat, although more direct research on this specific aspect would be beneficial. While bacterial counts in KNC were acceptable, future efforts should focus on improving hygiene in native chicken processing facilities to ensure microbial safety and preserve meat quality.
The sensory characteristics of broiler and KNC breast meat subjected to various freeze-thawing treatments were evaluated (Table 5). The appearance perception of fresh WRMD1 meat was significantly lower than that of broiler meat (p<0.05). However, meat from all three breeds (broiler, WRMD1, WRMD2) was similar in appearance after freeze-thawing except for SF-RT, SF-AT, and SF-WT treatments. The appearance acceptability of broiler meat was affected by freeze-thawing treatments, while KNC (WRMD1 and WRMD2) was not significantly impacted. Specifically, appearance scores for broiler samples exposed to SF-RT, SF-WT, QF-AT, and QF-WT treatments showed significantly lower scores (p<0.05) compared to fresh meat.
For taste perception, the fresh WRMD2 sample had a significantly lower score (p<0.05) than the broiler, while WRMD1 did not differ significantly from the broiler. In the freeze-thaw treatments, taste was generally not influenced by breed, except for the SF-MT and QF-WT treatments. The aroma and off-flavor characteristics were not affected by freeze-thaw treatment across all samples.
Breed influences juiciness in the fresh, SF-AT, and SF-WT samples. Tenderness was consistent across samples, except for the broiler QF-MT and WRMD2 SF-WT. Juiciness was generally unaffected by freeze-thaw treatment. The breed factor also had no significant impact on overall acceptability, except for the QF-WT treatment. The overall acceptability of broiler samples subjected to SF-WT, QF-AT, and QF-MT treatments showed significantly lower acceptability than that of fresh samples. Nevertheless, the remaining freeze-thaw treatments did not significantly affect the overall acceptability of the meat, regardless of breed.
The sensory characteristics of the frozen-thawed chicken meat in this study were similar to those of fresh meat, with little variation among the different breeds of chicken. While freezing and thawing can affect meat quality, the impact on sensory characteristics may be less pronounced than expected, especially with proper freezing and thawing techniques. Zhang et al. (2020) noted that thawing can affect texture characteristics like juiciness and hardness, but these changes may not be drastic enough to significantly alter overall sensory perception. Despite genetic differences between native and commercial broiler chickens, the basic muscle composition and structure remain similar across breeds, which can result in comparable sensory profiles. The sensory evaluation results in this study are consistent with the findings of Barido et al. (2022), who also found that sensory characteristics of frozen-thawed chicken meat were generally similar to those of fresh meat, with little variation between different breeds (broiler and KNC). However, these findings contrast with those described by Bae et al. (2014) and Leygonie et al. (2012), who reported that thawing resulted in loss of liquid and moisture in freeze-thawed meat as a result of muscle fiber shortening, leading to lower sensory scores. These differences in results may be due to different panelist profiles, while Bae et al. (2014) used experienced panelists, this study used undergraduate students representing typical consumers. As noted by Qi et al. (2021), a panelist’s ability to accurately discriminate between samples is greatly influenced by the amount of training and exposure to reference samples.
The results of this study offer significant insights into consumer preferences for KNC meat. The KNC’s superior water retention and rich flavor profile, which will be discussed further in the following section, may prove attractive to consumers seeking premium products. The minimal impact of freeze-thaw treatments underscores their suitability for frozen storage, which makes it appealing in modern supply chains. As interest in sustainability and locally sourced foods continues to grow, KNC’s unique traits could boost its market appeal as a distinctive, high-value product. However, it will be crucial to educate consumers about these distinct qualities to increase demand and establish a competitive market position.
Taste and flavor evaluations were conducted on selected freeze-thawing treatments, focusing on the analysis of fatty acids, nucleotide-related compounds, and VOCs. Specifically, the SF-RT and QF-RT treatments were selected for this analysis, with refrigerator thawing chosen as it is a commonly recommended method. Table 6 shows the composition of fatty acids in broiler and KNC chicken meat subjected to different freezing methods and then thawed in the refrigerator.
The profiles for total saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), unsaturated fatty acids (UFA), and polyunsaturated fatty acids (PUFA) were not significantly affected by the freeze-thaw treatment but were strongly influenced by the breed. The SFA content in broilers from fresh and SF-RT samples were lower than in both KNC breeds (p<0.05). Conversely, UFA and MUFA levels in broilers were significantly greater (p<0.05) in SFRT treatment group. On the other hand, the PUFA content in KNC (WRMD1 and WRMD2) showed higher significantly (p<0.05) than broilers in both SF-RT as well as QF-RT groups.
The disparity in fatty acid composition of broiler and native chicken has been attributed to genetic factors, dietary inputs, and the rearing environment. Native chickens typically have access to a diverse diet that includes plant material rich in PUFAs, specifically both omega-3 and omega-6 fatty acids. The free-range or less intensive rearing conditions of native chickens allow them to consume a more diverse range of foods, which can increase their PUFA content (Ali et al., 2021; Jayasena et al., 2013).
The results showed that fresh meat exhibited a greater concentration of fatty acids (p<0.05) compared to frozen-thawed meat. The higher fatty acid content in fresh samples may be explained by the freeze-thaw cycle causing oxidative degradation of fatty acids and cellular damage. This leads to increased loss of drippings, which contain several components including fatty acids, leading to their reduced content of thawed meat (Wereńska and Okruszek, 2022).
Whereas the fatty acid profile was strongly influenced by the breed. For example, the essential fatty acid linolenic acid showed significantly higher levels (p<0.05) in fresh WRMD1 than in the broiler meat. In addition, predominant fatty acids including stearic acid, palmitic acid, docosahexaenoic acid (DHA), and arachidonic acid were higher in KNC, especially WRMD1, than in broiler meat. These results are consistent with previous reports of higher levels of essential fatty acids in KNC than broilers (Barido et al., 2022; Lee et al., 2018).
Specific fatty acids are associated with taste. For example, DHA is perceived as sweet-bitter, umami flavor is associated with arachidonic acid, and oleic as well as linoleic acids are responsible for salty-sour tastes (Jayasena et al., 2013; Jayasena et al., 2015; Tang et al., 2009). In fresh and frozen-thawed state, DHA level was higher in both KNC breeds than in broilers. Arachidonic acid, which is related to umami taste, was significantly higher in both KNC breeds (WRMD1 and WRMD2) than in broilers for frozen-thawed samples. However, oleic acid content was lower in KNC than in broilers, in agreement with previous reports (Barido et al., 2022). Based on the fatty acid profiles of the KNC breeds reported results of this study indicate that these breeds are a valuable source of essential nutrients and have favorable taste characteristics.
The nucleotide-related compounds, including hypoxanthine, inosine, IMP, ADP, AMP, and ATP, were evaluated in broiler and KNC breast meat subjected to different freezing methods (Table 7). These compounds are crucial in the development of meat flavor, particularly contributing to umami taste and other sensory attributes (Felicia et al., 2023).
Hypoxanthine levels were primarily influenced by breed differences. WRMD2 exhibited the highest hypoxanthine content in the fresh state (9.71±0.618), which decreased significantly after freezing and thawing, particularly in the QF-RT group (7.76±0.933). This suggests that hypoxanthine is more stable in broiler meat during freezing and thawing, while it tends to decrease in WRMD2. Despite the reduction in hypoxanthine content in frozen-thawed meat, it remained comparable to the levels found in broiler meat (p>0.05). This finding is consistent with previous research that reported that there were no differences in hypoxanthine concentrations between broiler and KNC frozen-thawed meat (Choe et al., 2010).
IMP, a critical nucleotide for umami taste, showed significant variation among the samples. WRMD1 had the highest concentration of IMP in fresh as well as SF-RT states, and its IMP level significantly exceeded that of broiler meat in the QF-RT. Notably, IMP is recognized as an important flavor precursor in protein meat (Barido and Lee, 2021). Results suggest that breast meat derived from WRMD1 might have a favorable flavor profile, consistent with previous reports (Barido et al., 2022).
Inosine content was found to be higher in broiler meat than in KNC (WRMD1 and WRMD2) in the fresh, SF-RT and QF-RT groups. This result is consistent with previous findings reporting lower inosine levels in KNC meat than in broiler meat (Barido et al., 2022; Jayasena et al., 2014). In broiler meat, inosine levels in the QF-RT group were not significantly different (p>0.05) from fresh samples, suggesting that rapid freezing may better preserve inosine content compared to slow freezing.
AMP levels in WRMD1 remained relatively high across all treatments (Fresh, SF-RT, QF-RT), with no significant changes after freezing and thawing, indicating possible breed-specific stability in AMP content. ADP content was also mainly influenced by breed, with WRMD1 showing significantly higher ADP levels (p<0.05) than broiler meat in both the fresh and QF-RT sample groups. In KNC, ADP levels remained stable after freeze-thawing treatment, indicating better preservation of ADP in these breeds.
ATP levels showed minor variations among treatments and breeds, with no significant differences observed when comparing the breeds across all freeze-thawing treatments. Like ADP, ATP levels in KNC remained stable even after freeze-thaw treatment, indicating better preservation of ATP in these breeds.
Flavor, a combination of both taste and aroma, plays a critical role in whether consumers decide to repurchase meat products (Pittman et al., 2006). Typically, both specific VOCs and their overall classification largely determine how flavor and aroma are perceived (Troy and Kerry, 2010). Although previous studies have characterized the flavor of KNC, a detailed analysis of how different freeze-thaw treatments affect KNC flavor has not been conducted.
Altogether, 155 VOCs have been identified in the broiler and KNC, especially WRMD1 and WRMD2, as detailed in Supplementary Table S1. These VOCs fell into the categories of the following chemical classes: 26 aldehydes, 24 alcohols, 60 hydrocarbons, 9 ketones, 22 esters, and 14 others. The proportions of these chemical classes for each treatment group are illustrated in Fig. 2. Each of these groups contributes differently to meat flavor. Among the VOCs, hydrocarbons represented the largest proportion compared to other chemical families. This is consistent with findings from previous studies, which also identified hydrocarbons as the predominant VOCs in chicken meat (Dresow and Böhm, 2009).
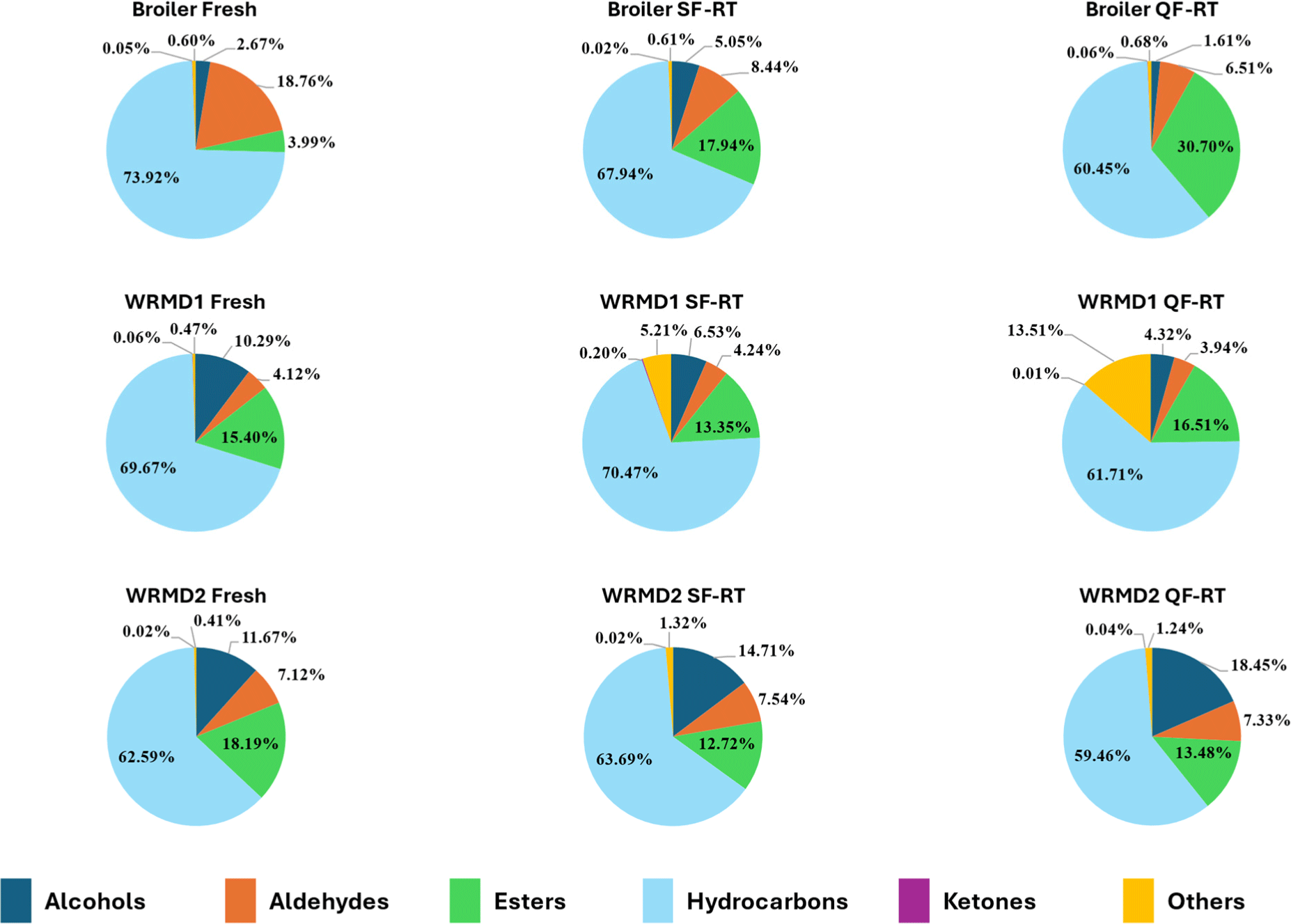
The alcohols group was slightly more abundant in both KNCs (WRMD1 and WRMD2) compared to the broiler. This group of VOCs may contribute to the distinct aroma of KNC. Similarly, prior research has identified alcohols as significant volatile components that contribute to the flavor of native chicken (Li et al., 2024).
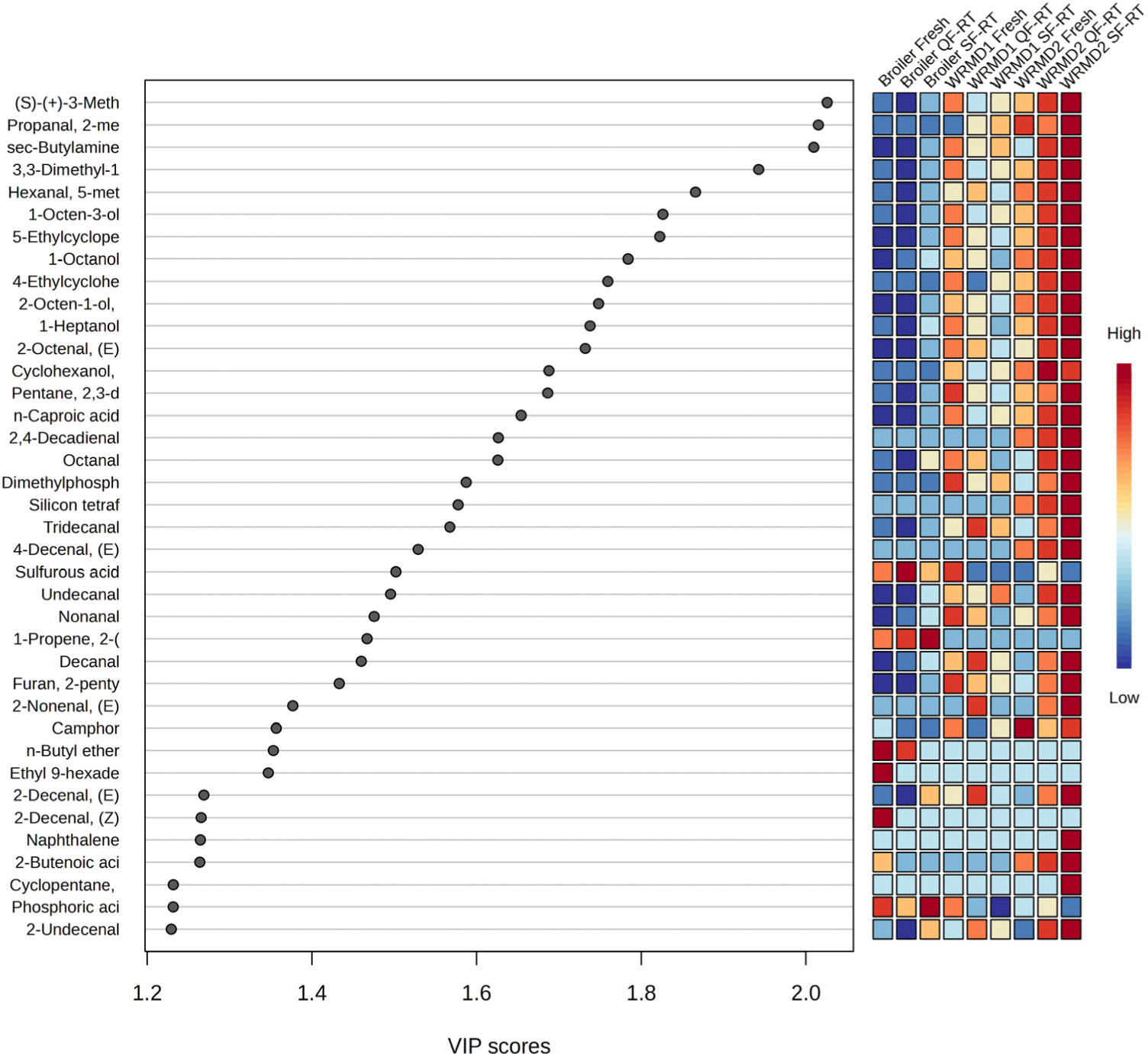
Fig. 1 presents a heatmap analysis of the VOCs in broiler and KNC breast meat subjected to different freezing methods. The color patterns in the heatmap indicate distinct differences between the broiler and the KNC. However, the factor of freezing methods did not show a notable impact on VOC profiles within the same breed. While freezing and thawing processes may alter meat’s physical texture, the VOC profiles remain largely influenced by the breed itself (Barido et al., 2022). This suggests that breed-specific characteristics, rather than freezing, are the primary determinants of meat flavor.
Variable importance in projection (VIP) score refers to how much each VOC contributes to differences between groups in a statistical model called PLS-DA. The VOCs with high VIP score (>1.2) were subjected to PLS-DA (Fig. 1). PLS-DA is a robust multi-variate statistics method used to analyze and classify data with numerous inter-correlated dependent variables (Ruiz-Perez et al., 2020). Variables with VIP scores greater than 1.2 were considered more effective indicators for distinguishing important VOCs across treatments (Anneke et al., 2024).
In the present investigation, 38 VOCs with VIP values greater than 1.2 were defined as key indicators to distinguish the effects of different breeds or freezing methods. The greater VIP score indicates a greater difference in the content of these variables between treatments, which has a stronger influence on the classification within PLS-DA plot (Tu et al., 2021). Among these compounds, several showed particularly higher VIP scores (>1.8), including (S)-(+)-3-methyl-1-pentanol, propanal, 2-methyl-, sec-butylamine, 3,3-dimethyl-1,2-epoxybutane, hexanal, 5-methyl-, 1-octen-3-ol, and 5-ethylcyclopent-1-enecarboxaldehyde. Consistent with previous research, (S)-(+)-3-methyl-1-pentanol and 1-octen-3-ol were identified as important VOCs that could serve as potential markers to distinguish meat from native chicken breeds (Shin et al., 2024). The (S)-(+)-3-methyl-1-pentanol is associated with cognac, cocoa, fusel, fruity and green aromas, while 1-octen-3-ol contributes to fishy, raw, earthy, oily, fungal, mushroom, chicken, and green aromas. 2-Methyl propanal has a flavor profile described as aromatic, with notes of chocolate, cocoa, dark, fat, and smoke. The sec-butylamine is associated with ammonia and fishy odors.
Given the VOC results in this study, it is suggested that compounds with VIP scores higher than 1.8 ((S)-(+)-3-methyl-1-pentanol, propanal, 2-methyl-, sec-butylamine, 3,3-dimethyl-1,2-epoxybutane, hexanal, 5-methyl-, 1-octen-3-ol, and 5-ethylcyclopent-1-enecarboxaldehyde) may serve as key biomarkers to discriminate broilers from KNC. In addition, no differences in flavor were observed between fresh and thawed meat, regardless of freezing treatment (quick or slow), highlighting the stability of flavor characteristics across different preservation methods.
Conclusion
This study highlights significant breed differences in the physicochemical, sensory, and flavor-related characteristics of KNC compared to broilers. KNC, particularly WRMD1, exhibited superior water retention and a more favorable fatty acid profile, with higher levels of essential and taste-related fatty acids. WRMD1 also showed the highest IMP concentration, contributing to a stronger umami flavor. Several VOCs, including (S)-(+)-3-methyl-1-pentanol and 1-octen-3-ol, were identified as potential markers for distinguishing KNC from broiler meat. Microbiological evaluations highlighted the need for improved hygiene management in KNC, while sensory analysis revealed no significant differences in overall acceptability between breeds. In general, the breed factor had a greater impact on meat quality than the freezing methods. While KNC, particularly WRMD1, showed advantages in certain quality parameters compared to broilers, improvements in hygiene and meat tenderness are still needed. Future studies should investigate the long-term effects of different freezing and thawing methods on the sensory and nutritional quality of KNC meat to provide a deeper understanding of how these processes impact overall meat quality over time. Consumer preference studies are also recommended to validate these findings and assess the market potential of KNC under different storage and preservation methods.













