Introduction
In recent years, the pet market, both domestically and internationally, has been expanding alongside an increase in pet-owning households. This growth is influenced by social phenomena such as aging, non-marriage, and urbanization, which have led to a rise in dual-income, no-kids (DINKs) households, nuclear families, and single-person households (Choe et al., 2023). As of 2022, one- and two-person households, which include DINKs, nuclear families, and single-person households, comprised 65% of the total household composition, significantly impacting pet ownership growth (Ministry of the Interior and Safety, 2023). According to the Ministry of Agriculture, Food and Rural Affairs (2023) “Announcement of 2022 public awareness survey on animal protection”, there were 6.02 million households with pets, representing 25.4% of all households. The pet market is anticipated to continue its growth trajectory, with projections showing an increase to 3.24 billion dollars in 2023, a 36.36% rise from 2.37 billion dollars in 2020 (Korea Rural Economic Institute, 2018).
The global pet market is broadly categorized into pet food, pet healthcare, pet services, and pet technology sectors. Among these, pet food dominated, accounting for approximately 70.94% (98.07 million dollars) of the total global pet market value of 138.24 million dollars as of 2020, marking it as the fastest-growing category (Gromek and Perek-Białas, 2022). In the Korean market, pet food represents 31.5% of the total pet-related industry and is growing at a rate of 5.4% annually (Korea Consumer Agency, 2021). Additionally, pet food constitutes 50.7% of the total pet care expenses, highlighting the expected growth in this sector (KB Management Research Institute, 2021).
The current trend in pet food is “pet humanization” which involves the anthropomorphization of pets. As part of this trend, pet foods such as “human grade”—made from ingredients safe for human consumption—and “raw food”—which consider the natural eating habits of pets—are being researched and developed (Ye et al., 2022). Additionally, many pet owners view their pets as family members, evidenced by neologisms such as “pet fam” and “pet me” in Korea, and “fur babies” and “fur family” in English-speaking countries (Rauktis et al., 2017; Seo, 2024). Consequently, pet food is developing from “feed” meant for herbivorous livestock to “food” intended for companion animals (Park et al., 2022). Therefore, for the pet food market to continue its expansion, it is crucial to manage protein-oriented animal feeds, distinct from traditional grain-oriented livestock feeds, and to establish effective non-thermal sterilization conditions for the distribution of pet food. Research on pet food forms, such as semi-wet, wet, and freeze-dried, which have better palatability than dry food, has significantly increased (Geary et al., 2023). However, semi-wet and wet pet foods, due to their higher moisture content, are more prone to microbial growth than dry pet foods (Watson et al., 2023). Moreover, health-conscious consumers (pet owners) prefer pet food without synthetic agents, which, while more natural, can deteriorate more quickly and require effective sterilization methods.
There are two main types of sterilization used to extend the shelf life of food: heat treatments such as warming, microwaves, and infrared radiation, and non-thermal treatments such as high-pressure processing (HPP), plasma, and radiation (Wang et al., 2023). Among these, heat treatments are the most common sterilization method, where food in various containers, such as pouches, cans, trays, and bottles, is heated directly or indirectly until the target sterilization temperature is reached (Barbosa-Cánovas et al., 2014). This process is crucial as it inhibits microbial activity, enhances flavor, and induces physical changes in food (Miri et al., 2008). However, heat treatment, typically at 121°C–140°C, can reduce quality characteristics such as flavor and color and destroy some nutritional components (Lazárková et al., 2011). Among nutritional components, proteins such as myoglobin are heat denatured at 30°C–35°C, and collagen begins denaturing at 58°C–65°C, disrupting the polypeptide structure (Vinnikova et al., 2019). Myoglobin, a plasma protein, changes color with heating—red at 60°C, pink at 60°C–70°C, and grayish-brown above 70°C–80°C—due to oxidation, significantly affecting the palatability of meat products (Han et al., 2024). Furthermore, the denaturation of collagen, a connective tissue protein, triggers protein hydrolysis, reducing its hardness; excessive heat denaturation can increase its toughness (Yu et al., 2017). Given the challenges associated with heat treatment, particularly for meat and fish raw ingredients rich in proteins, which are sensitive to heat, there is a transition to non-thermal sterilization technologies. These methods are being proposed as alternatives to traditional sterilization, especially for pet foods containing substantial amounts of animal protein (Lim and Ha, 2020; Shin, 2020).
Non-thermal sterilization is a technique that effectively minimizes the alteration of raw ingredients and inhibits microorganisms, incorporating physical treatments such as HPP, radiation, electric fields, and plasma, as well as chemical treatments using chemicals or cell wall-degrading enzymes (Song, 2020). With increasing consumer demand for high-quality foods that are healthful, safe from contamination, and free from synthetic products, non-thermal sterilization treatments are gaining attention as methods to ensure effective sterilization. Currently, non-thermal sterilization is primarily applied to raw food ingredients such as cereals, fruits and vegetables, seafood, and meat products, where traditional heating methods may reduce efficacy (Sunil et al., 2018). This technique halts the germination of grains, slows the ripening of fruits, inhibits the growth of parasites and microorganisms in food, and ultimately extends shelf life (Jan et al., 2017). For meat and fish, which are primary ingredients in pet food, microbial control is achieved through high-pressure washing, vacuum and modified atmosphere packaging, low-temperature systems, and disinfectant water sprays (Sohaib et al., 2016). However, meat and fish raw ingredients are more prone to external contamination and spoilage than vegetable ingredients, necessitating effective sterilization methods to eradicate harmful microorganisms completely (Molins et al., 2001). In recent years, non-thermal sterilization methods like HPP, plasma, and radiation have been explored for various meat and fish raw ingredients used in raw pet food (Lee et al., 2023; Neshovska et al., 2023; Serra-Castelló et al., 2023; Yadav and Roopesh, 2020).
The Association of American Feed Control Officials (AAFCO) stipulates that dry pet food should contain 18% to 22.5% crude protein and 5.5% to 8.5% crude fat (Dodd et al., 2021). Consequently, the main ingredients of domestically and internationally distributed pet food typically include meat products such as chicken, beef, pork, and duck, and fish products such as mackerel, tuna, and salmon, along with by-products from these sources (Montegiove et al., 2021). Unlike advanced countries in the pet food industry like the United States and Europe, Korea lacks a standard for the non-thermal sterilization of pet food, necessitating a comprehensive review of existing research to establish such a standard.
High-Pressure Processing
HPP, also known as high hydrostatic pressure or ultra-high-pressure, is a technology that can effectively sterilize harmful microorganisms while maintaining the quality characteristics of food, such as flavor, color, and nutritional content (Seo et al., 2014). This method applies Le Chatelier’s chemical equilibrium principle, where the volume and number of molecules in food decrease under pressure, leading to microbial cell membrane disintegration, protein denaturation, and sterilization (Renaud et al., 2022). Additionally, cold isostatic pressure involves pressurizing food with a liquid medium at low or room temperature (Dalai and Sahu, 2010).
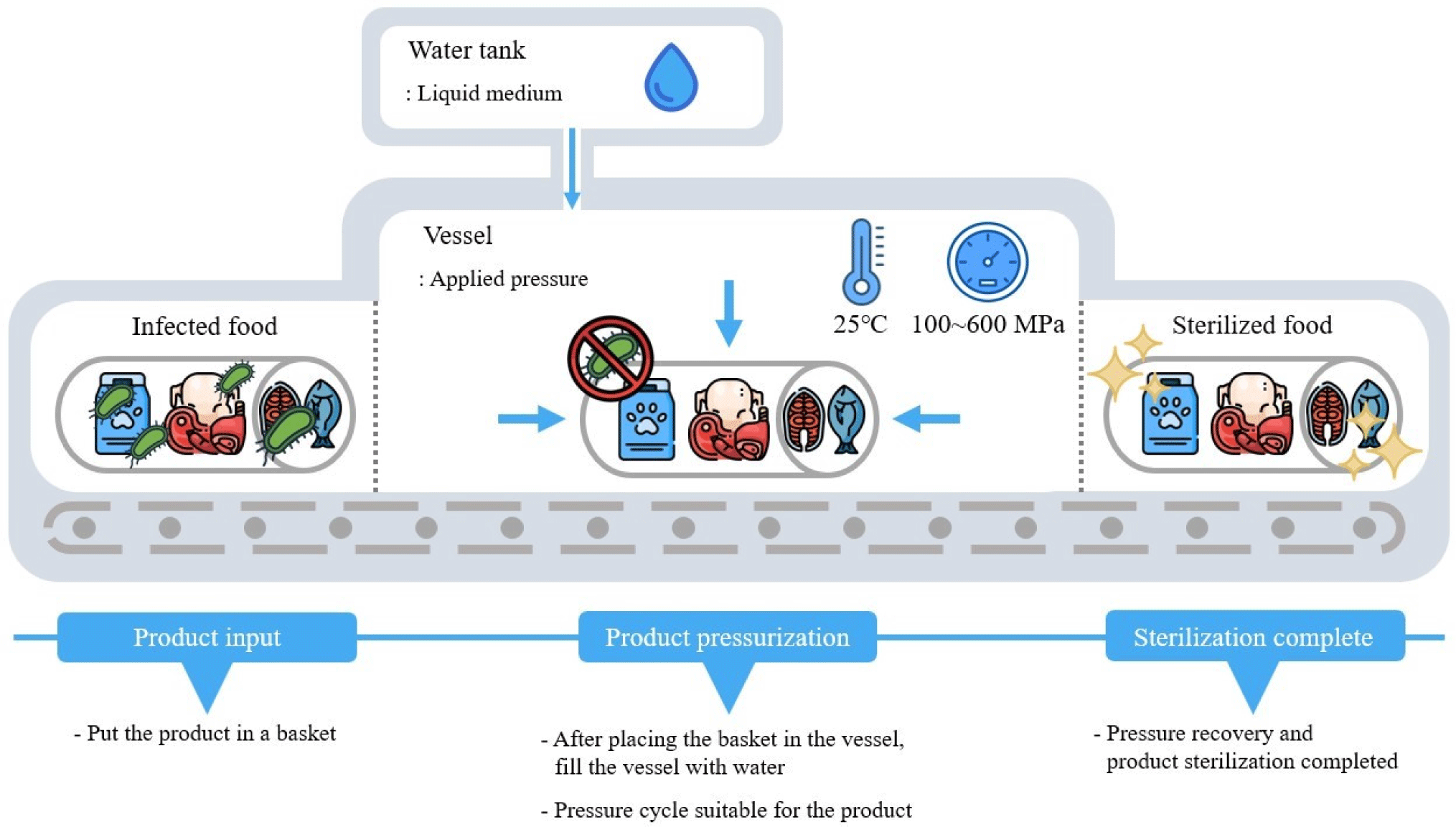
During HPP, covalent bonds are less affected by pressure, while weaker electrostatic and hydrophobic bonds undergo structural deformation due to pressure (Campus, 2010). This deformation destroys the cellular structure of microorganisms and inactivates enzymes, but does not affect small molecular size vitamins and flavor compounds, thus, preserving nutritional substances (Albert et al., 2021). Moreover, HPP has the advantage of minimizing food deformation since there is no heat involved, providing uniform and instantaneous treatment to food through a liquid medium at pressures ranging from 100 to 600 MPa, resulting in minimal deformation in the size and shape of food (Li and Farid, 2016). Consequently, HPP can be applied not only to raw food products but also to packaged finished products, making it a highly adaptable technology in the food industry (Koutsoumanis et al., 2022).
HPP has applications across various fields including food, pharmaceuticals, and medicine, predominantly within the food industry. This application in the food sector began when Hite (1899) observed a reduction in microorganism levels in milk and meat subjected to high-pressures of 500–600 MPa (Torres and Velazquez, 2005). Throughout the 20th century, research into HPP technology expanded in countries like the United States, the United Kingdom, and Japan, leading to the development of HPP equipment suitable for sterilizing food by eliminating harmful microorganisms. Fig. 1 presents a schematic diagram of the structure and operational process of a currently commercialized HPP device. The container, liquid medium, and pressure range of the device have been standardized, facilitating the wider use of HPP in food processing.
As a result, a variety of high-pressure processed and pasteurized foods, including meat products, seafood, juices, jams, and purees, have been commercialized. The primary microorganisms affected by HPP include Listeria spp., and Campylobacter spp., making HPP particularly advantageous for sterilizing meat and fish raw ingredients, which are highly susceptible to microbial contamination (Campus, 2010). However, it has been reported that foods rich in nutrients, such as carbohydrates, proteins, fats, and salts, or those with low water activity, can exhibit microbial protective effects against HPP (Govaris and Pexara, 2021). Therefore, microbial sensitivity to pressure can vary depending on the state of the food, highlighting the need to establish specific sterilization conditions for each type of food.
Countries like the United States, the United Kingdom, and Canada, which are advanced in the development of HPP, are actively implementing HPP technology in the food industry and have established policies for foods processed by HPP. The U.S. Food Safety and Inspection Service (FSIS), the U.K. Food Standards Agency (FSA), Canada’s Health Canada, and the Food Standards Agency of Australia and New Zealand (FSANA) have all set policies for the application of HPP in foods (Huang et al., 2017). In contrast, South Korea lacks specific policies and standards for foods processed by high-pressure, underscoring the necessity to establish precise pressure levels and durations to effectively sterilize microorganisms during HPP application.
Table 1 reviews prior literature on the application of HPP sterilization to meat and fish raw ingredients, which are the primary animal proteins used in pet food. This table illustrates that HPP, predominantly utilized for sterilizing meat and fish products, can achieve the five-log reduction in microorganisms mandated by international regulatory bodies by processing at 400 to 600 MPa at temperatures of 4°C or 25°C in under 10 min (Bolumar et al., 2021). Key control points for effective sterilization include processing temperature, pressure, and time. Notably, lower temperatures, such as 4°C, have proven to be more effective in eliminating microorganisms than temperatures around 25°C, with the effectiveness of the microbial disinfaction increasing with longer processing times. Additionally, the processing pressure impacts the physical state of proteins in the sample and myoglobin oxidation (Pou, 2021).
| Raw ingredients | Processing conditions | Experimental results | Reference |
|---|---|---|---|
| Chicken (chicken leg) | 0.1, 300, 600 MPa (15±3°C, 5 min) | - Total microorganisms were reduced to undetectable levels when treated at 300 and 600 MPa. - When treated at 300 and 600 MPa, total microorganisms decreased by 3 and 4 Log CFU/g, respectively, compared to the control after 7 days of storage. - Treatment at 300 and 600 MPa increased CIE L* and decreased CIE a* and CIE b*. - Treatment at 300 MPa had no effect on thiobarbituric acid reactive substances (TBARS) and protein oxidation. - The addition of phosvitin during ultra-high pressure treatment increased TBARS, protein oxidation, and microbial suppression. |
Jung et al. (2012) |
| Total microorganisms (TSA, 37°C, 48 h) | |||
| Storage conditions 0, 3, 7 days |
|||
| Chicken (chicken breast) | 500 MPa (18°C–20°C, 10 min) | - Brochothrix thermosphacta, Pseudomonas spp., Enterobacteriaceae, Lactobacillus, and yeasts and molds were detected at 1 Log CFU/g when treated at 500 MPa at 4 and 12°C. - The pH increased compared to the control when treated at 500 MPa. |
Argyri et al. (2018) |
| Total microorganisms (PCA, 30°C, 48–72 h) Lactobacillus (MRS, 30°C, 48–72 h) Yeast and mold (DRBC, 25°C, 48–72 h) Gut bacteria (VRBG, 37°C, 24 h) Pseudomonas spp. (pseudomonas agar base, 25°C, 48 h) Salmonella enteritidis (XLD, 37°C, 16–18 h) |
|||
| Storage conditions 0, 5, 10 days 4°C, 12°C |
|||
| Pork | 50, 100, 200, 300, 400, 500 MPa (15 min) | - Total microorganisms decreased to less than 3 Log CFU/g when treated at over 400 MPa. - Total microorganisms of pork treated at 500 MPa for 1, 5, and 15 min, decreased from approximately 3 Log CFU/g in the control to 2.17, 1.77, and 2.33 Log CFU/g, respectively. - The pH of the pork increased with higher treatment pressure and longer treatment time compared to the control. When treated at 500 MPa for 15 min, the pH was approximately 5.96, which was higher than the control (approximately pH 5.4) and other treatment groups (approximately pH 5.6–5.9). |
Sazonova et al. (2017). |
| Total microorganisms (NA, 30°C, 48 h) | |||
| Pork Fuji (cooked O) Smoked pork loin (cooked X) |
300, 400, 500, 600 MPa (10 to 60 min) | - Cooked pork traditional ham treated at 600 MPa for 10 min showed no detection of coliform, Enterococci, or acidophilic bacteria during the 8-week storage period. - Cooked pork traditional ham treated at 300 MPa or higher for more than 10 min showed increased CIE a*, CIE b*, and shear force, and a decrease in CIE L* compared to the control. |
Karłowski et al. (2002) |
| Escherichia coli Enterococci Mesophilic and thermophilic bacteria Eosinophils |
|||
| Storage conditions 0, 4, 6, 8 week 4°C to 6°C |
|||
| Beef (breast, pectoral) | 400, 600 MPa (35°C, 45°C, 55°C, 20 min) | - Beef treated at 400 MPa or higher at 35°C showed increased pH, CIE L*, CIE b*, and cooking loss, and a decrease in CIE a*. - Cooking loss increased as the treatment pressure increased. |
McArdle et al. (2011) |
| Total microorganisms (PCA, 30°C, 72 h) Lactobacillus (MRS, 37°C, 24 h) Gut bacteria (VRBG, 30°C, 24 h) Listeria Salmonella Campylobacter |
|||
| Storage conditions 0, 7, 15, 30 days | |||
| Sliced beef | 300, 450, 600 MPa (5 min) | - Treatment with pressures of 300, 450, and 600 MPa, including 2% citric acid and 2% salt solution, reduced the growth of E. faecium by 1, 4, and 6 Log cycles, respectively. Increasing the citric acid content was effective in inhibiting E. faecium. - Treatment with pressures of 300–600 MPa, including 2% citric acid and 2% salt solution, reduced the growth of L. innocua by 6 Log cycles. Increasing the salt concentration was effective in inhibiting L. innocua. - L. innocua is more sensitive to high-pressure treatment than E. faecium. When treated at 450 MPa with a solution containing 1% salt and 2% citric acid, L. innocua was reduced by 7 Log cycles. |
Rodrigues et al. (2016) |
| Listeria innocua (Oxford ager, 37°C, 48 h) Enterococcus faecium (Enterococcosel agar, 37°C, 48 h) |
|||
| Salmon, cod, mackerel | 0.1, 200, 500 MPa (2 min) | - Cod and mackerel treated at 500 MPa showed total aerobic bacteria reduced to undetectable levels. - All treatment groups showed an increase in TBARS and pH compared to the control when treated at 200 MPa or higher. |
Rode and Hovda (2016) |
| Thermophilic microorganisms (Long & Hammer agar, 15°C, 5–7 days) Hydrogen sulfide-producing microorganisms (Iron ager, 20°C, 3–4 day) |
|||
| Storage conditions Cod: 0, 7, 11, 14, 22 days Salmon: 0, 7, 11, 15, 18, 21, 26 days Mackerel: 0, 4, 7, 11, 14, 19 days |
Approximately 30% of processed foods treated with high-pressure sterilization are meat products. The sterilization of meat products using high-pressure is already widespread, as the quality and sensory properties of meat products are relatively stable under high-pressure conditions compared to fresh meat (Bolumar et al., 2021). However, unlike processed meat products, raw meat is influenced by various factors such as the characteristics of the meat, and the temperature, pressure, and duration of processing. For example, chicken or fish meat, which is softer than pork or beef, tends to undergo protein gelation when pressurized at 200 to 300 MPa (Chen et al., 2018). Additionally, the color of meat changes more sensitively in red meats rich in myoglobin. Pressurizing above 300 MPa disrupts the physical structure of myoglobin, accelerating oxidation, which results in increased brightness and reduced CIE a* of the meat; however, these color changes induced by HPP treatment tend to lessen over extended storage periods (Bak et al., 2017).
Therefore, it is necessary to compare the sterilization efficiency and quality between raw ingredients and finished products and to investigate the suitable HPP conditions for microorganisms with high contamination potential depending on the type of product (dry, wet, semi-wet, freeze-dried, etc.).
Plasma
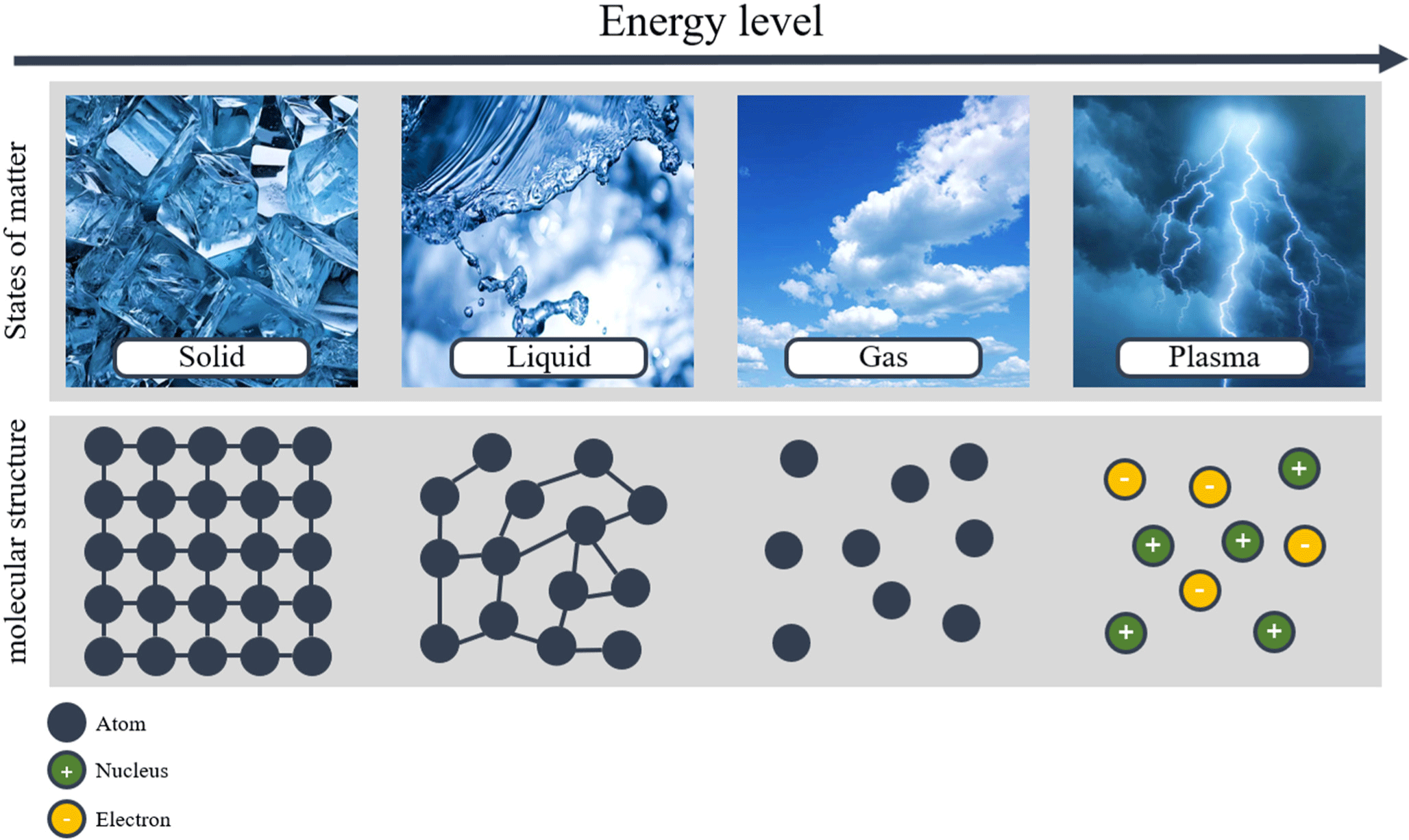
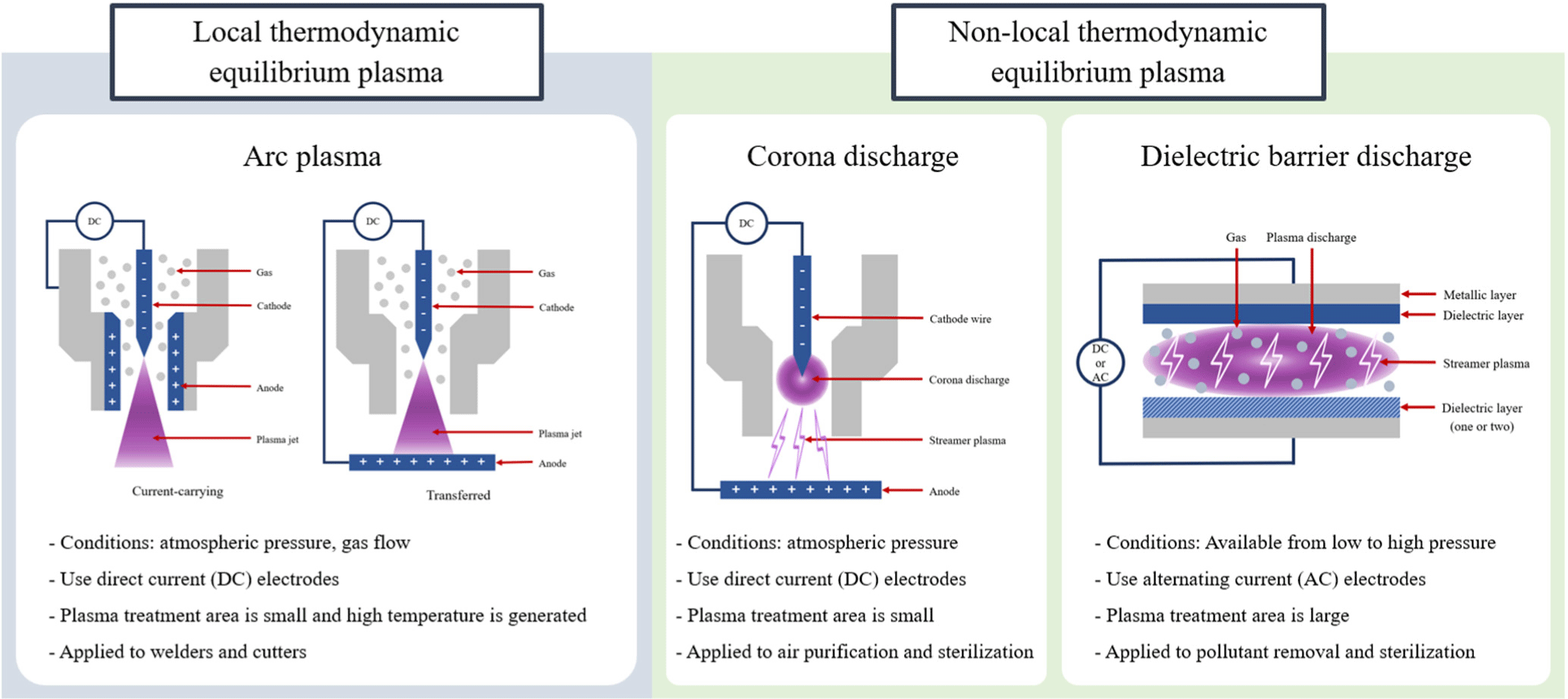
Plasma is a fourth state of matter in which ions and electrons, separated by high energy, are either in an energy equilibrium or non-equilibrium state, as depicted in Fig. 2. It consists of ions, electrons, free radicals, and ultraviolet radiation, serving as the source for phenomena such as lightning, aurora borealis, neon signs, and fluorescent lights (Jeon et al., 2020). Plasma can be categorized into hot plasma (equilibrium plasma; arc discharges) and cold plasma [non-equilibrium plasma; corona and dielectric barrier discharges (DBDs)], according to the equilibrium state of ions and electrons, as illustrated in Fig. 3. Cold plasma is also referred to as non-equilibrium plasma because the electron temperature is higher than the overall gas temperature (Fiebrandt et al., 2018).
Furthermore, low-temperature plasmas are produced and sustained in an ionized gas state by arc discharge, corona discharge, and DBD at low-pressure (depressurization) or at normal pressure (atmospheric pressure; Birania et al., 2022). Arc discharge is characterized by generating high-temperature plasma in a very confined area at normal pressure, while corona discharge also generates plasma at normal pressure, resulting in a limited processing area, albeit with comparatively less heat generation than arc discharge (Dalvi-Isfahan et al., 2023). Conversely, dielectric barrier plasma is created by attaching one or two dielectric plates to two parallel metal plates connected to direct current or alternating current electrodes, producing less heat and covering a larger processing area (Nasiru et al., 2021).
Among the low-temperature plasma technologies, normal pressure plasma is predominantly utilized in conjunction with low-temperature sterilization. However, controlling the plasma production rate is challenging, and temperature increases can occur due to excessive energy input (Domonkos et al., 2021). In contrast, low-pressure plasma is ideal for non-thermal sterilization as it allows for a controlled rate of plasma generation and minimizes heat production (Fiebrandt et al., 2018). Alongside low-pressure plasma, plasma-activated water (PAW) is also being explored as a non-thermal sterilization method for meat, fruits, and vegetables (Sammanee et al., 2022).
Microbial sterilization by plasma has been explored since the mid-1990s, though its practical applications in the food industry commenced in the early 2000s (Li and Farid, 2016). Plasma sterilization is particularly advantageous because it does not involve chemical treatments or leave residues. Its uses in the food industry have expanded to include sterilizing microorganisms, removing contaminants, extending shelf life, inactivating enzymes, eliminating toxins, enhancing food packaging, and treating wastewater (Hati et al., 2018).
The mechanism of microbial sterilization by plasma primarily involves DNA modification through ultraviolet rays emitted by the plasma (UV inactivation), the release of volatile substances from chemical bond sterilization (photodesorption), and the adsorption reaction of reactive compounds (etching; Mravlje et al., 2021). This method is effective against harmful pathogens such as Escherichia coli, Salmonella spp., Listeria spp., noroviruses, and hepatitis viruses. These pathogens are commonly found in environments associated with animal slaughter, making them critical targets for sterilization in the food processing industry.
Plasma technology is frequently applied to meat products such as chicken, pork, and beef. It efficiently sterilizes various harmful substances and microorganisms while minimally impacting quality characteristics like color and texture, making it a valuable technology for ensuring safety in the food supply (Misra and Jo, 2017).
Table 2 is a prior study of plasma sterilization technologies applied to meat and fish raw ingredients, which are the animal proteins most commonly used in pet food. The table shows that plasma, primarily utilized for sterilizing meat and fish, operates under normal pressure or low-pressure at temperatures below 44°C. This setup minimizes protein modification and is achieved through the use of dielectric barrier plasma or PAW (Sen and Mutlu, 2013).
| Raw ingredients | Processing conditions | Experimental results | Reference |
|---|---|---|---|
| Chicken (chicken breast) | Normal pressure plasma (10 s, 30 s, 1 min, 2 min, 3 min) Gap: 1.0–1.5 cm Voltage: 6.0–11.0 kV Frequency: 23.0 to 38.5 kHz He: 5.0 L/min O2: 50, 100 mL/min |
- The thicker and rougher the surface of the sample, the less effective the plasma treatment. - The sterilization of L. innocua on skinless chicken breast has been reported with a gap of 1 cm, voltage of 8–11 kV, frequency of 30–38.5 kHz, and O2 flow rate of 0–25 mL/min for 4 min. - The sterilization of L. innocua on chicken breast with skin has been reported with a gap of 1 cm, voltage of 7 kV, frequency of 38.5 kHz for 3 min, and with a gap of 1 cm, voltage of 8 kV, frequency of 38.5 kHz for 30 s. - Voltages above 9 kV caused overheating of the transformer, and stable operation was achieved at a frequency of 23 kHz. |
Noriega et al. (2011) |
| Total microorganisms (TSA, 30°C, 24 h) Listeria innocua (TSB, 30°C, 48 h) |
|||
| Chicken/pork (chicken breast/ pork belly) | Plasma-activated water (PAW) (immersion, 25.5°C, 15 min) Voltage: 125 W, 15 kV Frequency: 50 Hz 60 ppm H2O2, 500 mL |
- Treatment of pork and chicken with hydrogen peroxide PAW resulted in a greater reduction of C. jejuni compared to other microorganisms. - Hydrogen peroxide PAW treatment on the surface of pork resulted in an increase in pH, CIE L*, temperature, and water activity, and a decrease in CIE a* and CIE b*. - Hydrogen peroxide PAW treatment on the surface of chicken resulted in an increase in pH and CIE a*, and a decrease in CIE L*, CIE b*, and temperature. |
Sammanee et al. (2022) |
| Salmonella typhimurium (XLD, 37°C, 24 h) Campylobacter jejuni (mCCD, 41.5°C, 48 h) Escherichia coli (ECD, 44°C, 24 h) Staphylococcus aureus (BPA, 37°C, 48 h) Pseudomonas aeruginosa (GSP, 30°C, 48 h) |
|||
| Storage conditions 0, 3, 7, 10 days 4°C to 6°C |
|||
| Pigs (sirloin) | Vacuum plasma (5, 10 min) Frequency: 20–100 kHz |
- When treated with helium gas and plasma, total microorganisms, psychrophilic bacteria, yeasts, and molds were detected at 2.49, 2.56, and 1.35 Log CFU/cm2, respectively. - When treated with argon gas and plasma, total microorganisms, psychrophilic bacteria, yeasts, and molds were detected at 3.13, 3.13, and 1.86 Log CFU/cm2, respectively. - When treated with nitrogen gas and plasma, total microorganisms, psychrophilic bacteria, yeasts, and molds were detected at 5.02, 5.09, and 3.37 Log CFU/cm2, respectively. - pH showed no significant effect when treated with vacuum plasma. |
Ulbin-Figlewicz et al. (2015) |
| Total microorganisms (PCA, 30°C, 72 h) Total yeast and mold (SDAC, 25°C, 5 day) |
|||
| Beef (10 g) | PAW (1 mL spray, 0, 5, 10, 20, 30 min) Voltage: 10 kV Frequency: 8 kHz 40 mL deionized water |
- A 5 min treatment with PAW resulted in a pH decrease of 3.7 compared to the control. - Longer treatment with PAW led to an increase in volatile basic nitrogen (VBN) and thiobarbituric acid reactive substances (TBARS). - PAW treatment resulted in a decrease in CIE a* after 4 days of storage. |
Zhao et al. (2018) |
| Total microorganisms (PCA, 37°C) Total bacteria (PCA, 27°C) |
|||
| Storage conditions 6, 12, 24, 48, 96, 192 h, 4°C |
|||
| Mackerel (fillet) | PAW (immersion, 25°C, 10 min) Voltage: 550 W Frequency: 25 kHz 200 ppm CH3COOH 30 mL |
- A 10 min treatment with plasma-activated peracetic acid (PA-PAA) resulted in a 3.8 Log CFU/g reduction in total microorganisms. - PA-PAA treatment had no significant effect on color and TBARS. |
Zhao et al. (2021) |
| Total microorganisms (30°C, 48 h/6.5°C, 10 day) E. coli (MacConkey, 37°C, 24 h) L. innocua (Oxford, 37°C, 24 h) Pseudomonas fluorescens (CFN, 30°C, 24 h) |
TSA, tryptic soy agar; TSB, tryptic soy broth; XLD, xylose lysine desoxycholate agar; mCCD, modified charcoal cefoperazone deoxycholate agar; ECD, Escherichia coliform detection agar; BPA, Baird Parker agar; GSP, glutamate starch phenol red agar; PCA, plate count agar; SDAC, sabouraud dextrose agar with chloramphenicol; CFN, cetrimide fucidin nalidixic acid agar.
Non-thermal plasma sterilization applied to chicken has been mainly studied for chicken breasts and thighs, using dielectric plasma (Lee et al., 2020; Zhao et al., 2022) or immersion in PAW (Kang et al., 2022; Qian et al., 2021; Sammanee et al., 2022). However, chicken with skin has a non-uniform surface, which reduces the microbial inactivation effect compared to chicken with a uniform surface (Noriega et al., 2011). Effective microbial inactivation of chicken skin can be achieved with a radiation gap of 1.0 cm, a voltage of 7.0 kV, and a frequency of 38.5 kHz for 3 min, or a 30 s exposure at 8.0 kV. Conversely, skinless chicken breasts showed negative results for Listeria at a radiation distance of 1.0 cm, voltages of 8.0–11.0, and frequencies of 38.5–30.0 kHz, suggesting that higher voltage and frequency are effective in sterilizing Listeria in chicken.
Other factors affecting the effectiveness of plasma sterilization include exposure time, flow rate, moisture, temperature, and gas composition. Regarding atmospheric composition, nitrogen, argon, and helium are the most commonly used gases (Ulbin-Figlewicz et al., 2015). The effects of vacuum dielectric plasma sterilization of pork loin with these different gas compositions showed that helium had the highest microbial inactivation rate, with yeast and mold showing higher disinfection effects than total microorganisms and thermophilic bacteria. Plasma with different gas compositions did not significantly affect the pH of the sample, indicating that it does not cause significant changes to the original product. Despite these advantages, dielectric barrier plasma has the drawback of requiring large and expensive equipment for large-scale treatment. Consequently, PAW is being researched as a more feasible alternative (Du et al., 2022).
PAW is produced by passing an electrode through the liquid in a water tank by using a plasma generator, and this PAW is treated by spraying it onto the surface of the sample or immersing the sample in it (Hadinoto et al., 2023; Lotfy and Khalil, 2022; Qian et al., 2022). This type of PAW is typically produced using hydrogen peroxide, perchloric acid, sodium hypochlorite, and acetic acid. The typical application conditions for PAW involve a 10 min treatment at temperatures around 25°C. Studies have shown that hydrogen peroxide plasma treatment of pork and chicken is highly effective against pathogens such as Campylobacter and Salmonella enteritidis, but not against Pseudomonas (Sammanee et al., 2022). The pH and chromaticity measurements indicate variations between deep and surface treatments, suggesting that the use of PAW should be carefully managed to avoid adversely affecting the palatability of the food. When applied to mackerel, PAW also resulted in a decrease in pH without significant changes in chromaticity.
Unlike dielectric barrier plasma, PAW can be used in large quantities either through immersion or spraying. However, it is important to assess the effects of different types of PAW on the palatability of food to ensure suitability. Therefore, while plasma sterilization of pet food ingredients and finished products with uniform surfaces is typically superior in maintaining quality and sensory properties when treated with dielectric barrier plasma at around 25°C, this method is more costly compared to treatments using PAW. Consequently, comparative studies between different plasma treatment methods for pet food ingredients and finished products are essential to determine the most cost-effective and quality-preserving sterilization technique.
Radiation
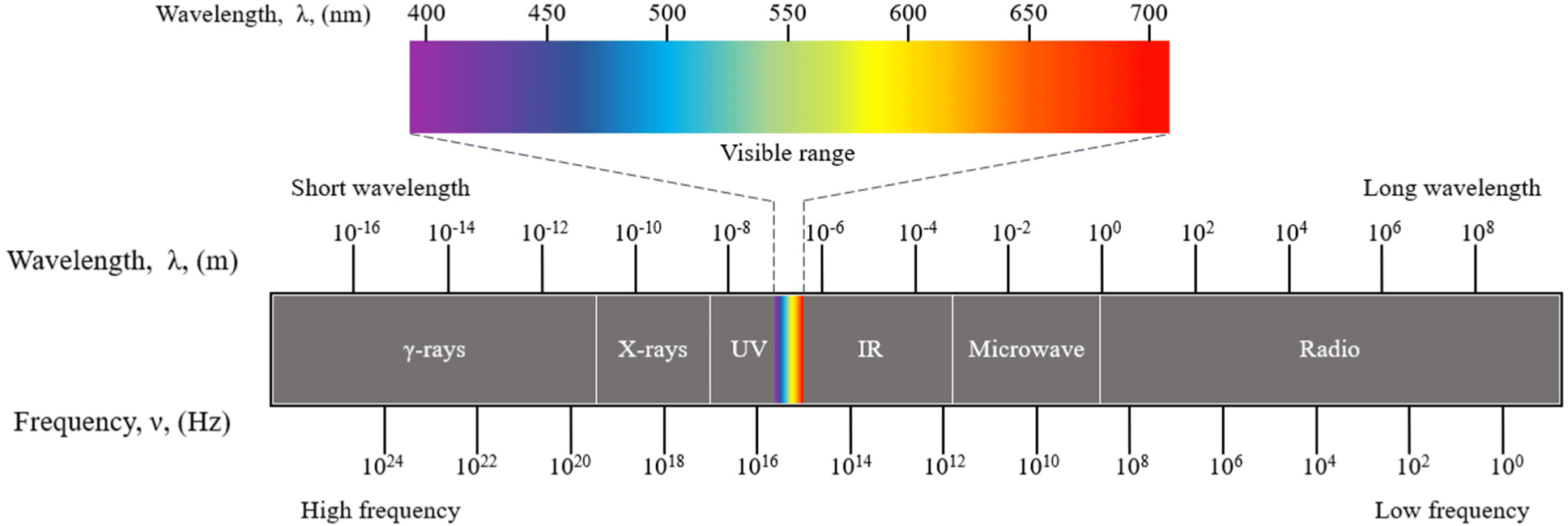
Radiation is categorized into natural and artificial types, as illustrated in Fig. 4. Artificial radiation includes alpha (α), beta (β), and gamma (γ) rays emitted from radioactive isotopes, mechanically generated X-rays, electron beams generated by electron accelerators, and neutron rays produced by nuclear reactors (Shahi et al., 2021). Ionizing radiation, which has the property of ionizing the atoms or molecules of a substance through which it passes, results in the formation of ions. This category includes alpha rays, gamma rays, electron beams, ultraviolet rays, and neutrons (Akhila et al., 2021).
Food irradiation has been consistently raising safety concerns, and in response, relevant international organizations such as Food and Agriculture Organization (FAO), International Atomic Energy Agency (IAEA), and World Health Organization (WHO) have demonstrated the safety of irradiating food with less than 10 kGy of gamma rays, X-rays, electron beams, and ultraviolet rays (UV; Venugopalan and Suprasanna, 2022). This is because radiation leaves no chemical residues and is largely unaffected by environmental conditions (Jildeh et al., 2021). Gamma rays and X-rays, known for their strong penetrative power, allow for the treatment of opaque samples or packaged finished products, which helps prevent secondary contamination that could occur during repackaging after sterilization (Mshelia et al., 2023).
Gamma rays are emitted radially around the radiation nucleus, while X-rays and electron beams involve the use of electricity to generate artificially accelerated electrons, focusing on sterilizing concentrated areas. In contrast, UV and electron beams, which have weaker penetrating power than gamma and X-ray rays, can be precisely controlled via a power source, offering advantages in terms of process control, rapidity, accuracy, and efficiency. Among the ultraviolet spectrum, UV is categorized into UVA (315–400 nm), UVB (280–315 nm), and UVC (200–280 nm), with UVC being the type used for microbial sterilization (Shahi et al., 2021).
These radiation types are employed in various applications, such as inhibiting the germination and rooting of agricultural products, sterilizing pathogens and parasites, controlling the ripening of agricultural products, and extending their shelf life.
The use of radiation in the food industry began in 1895 with Roentgen’s discovery of X-rays and the subsequent recognition of their potential to sterilize microorganisms in food. In 1921, radiation was first utilized in the United States to sterilize trichinosis in pork (Jildeh et al., 2021). Initially, the high cost of radiation equipment limited its practical use. However, by 1950, the mass production of radioactive materials facilitated comprehensive research (Demir et al., 2019). Radiation sterilizes food either directly by damaging microbial nucleic acids, which inhibits nucleic acid synthesis and microbial division, or indirectly. Indirect sterilization occurs when radiation generates decomposition products such as free radicals in water molecules; these highly reactive radicals combine with oxygen to form oxidizing agents, which damage microbial cell membranes and thus sterilize the microorganisms (Li and Farid, 2016).
Given the varying susceptibility of microorganisms to radiation, its application is categorized into complete sterilization, pathogenic microorganism sterilization, and partial sterilization (Rosario et al., 2021). Complete sterilization involves high-dose radiation (30–50 kGy) targeting endospore-producing microorganisms such as Bacillus spp. and Clostridium spp. This level of radiation is employed for foods requiring high safety standards such as canned food, hospital food, space food, sports food, and aseptic feeds for laboratory animals. Pathogenic microorganism sterilization, which uses a radiation dose of 1 to 10 kGy, targets harmful microorganisms in food. Partial sterilization aims to reduce the microbial load in food with radiation doses ranging from 0.5 to 10 kGy to extend its shelf life and refrigeration period.
The advantages of radiation include shorter processing times compared to heating, no chemical residues, and easier handling (Yang et al., 2023). However, certain radiation doses can lead to undesirable changes such as discoloration and softening of fruits, oxidation of meat products, and consumer resistance due to concerns about radiation exposure (Ahn et al., 2013; Shahbaz et al., 2016). Therefore, determining the appropriate type and dose of radiation for meat-based pet food is crucial.
Table 3 presents a prior study on radiation sterilization technology applied to meat and fish products, which are primary sources of animal proteins in pet food. It demonstrates that gamma rays and electron beams below 10 kGy are the most prevalent forms of radiation used for the sterilization of meat and fish, achieving general sterilization at doses above 6 kGy across all cultures. However, doses around 3 kGy were found to produce excessive peroxides and volatiles, leading to off-flavors and other issues affecting palatability (Farkas, 1998). Consequently, research is being conducted on the impact of radiation on samples supplemented with natural or synthetic antioxidants.
| Raw ingredients | Processing conditions | Experimental results | Reference |
|---|---|---|---|
| Chicken (machine-boned chicken) | Gamma rays (0.30, 0.60, 0.90, 1.20, 1.80, 2.70, 3.60 kGy) | - Gamma irradiation treatment at 20°C exhibited a higher inhibitory effect on S. typhimurium compared to –20°C and 0°C. - Atmospheric pressure conditions showed a higher inhibitory effect on S. typhimurium compared to vacuum conditions. - When irradiated with 3.6 kGy at 20°C, Salmonella was detected at 2.75 Log CFU/g under atmospheric pressure conditions and 3.06 Log CFU/g under vacuum conditions. - When irradiated with 3.0 kGy at –20°C, Salmonella was detected at 3.93 Log CFU/g under atmospheric pressure conditions and 4.29 Log CFU/g under vacuum conditions. |
Thayer and Boyd (1991) |
| Salmonella typhimurium (TSA, 35°C, 24 h) | |||
| Chicken (chicken patty) Guava leaf extract |
Gamma rays (2 kGy) | - Under atmospheric pressure conditions, total phenol content (TPC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and ferric reducing antioxidant power (FRAP) were lower, while total volatile basic nitrogen (TVBN), thiobarbituric acid reactive substances (TBARS), peroxide value (POV), total anaerobic bacteria, and fecal coliforms were higher compared to vacuum conditions. - As the concentration of guava leaf extract increased, the detection of total anaerobic bacteria and fecal coliforms decreased. - Treatment with 2 kGy resulted in an increase in TPC, DPPH, FRAP, CIE L*, CIE a*, and CIE b* compared to the control, while TVBN, TBARS, and POV decreased. |
Sadiq et al. (2023) |
| Total microorganisms (PCA) | |||
| Storage conditions 0, 5, 10 days |
|||
| Pork (ground pork, 30 g) | Gamma rays (5 kGy) | - The control treated with 5 kGy showed no detection of P. aeruginosa and L. casei. - After irradiation treatment and inoculation with P. aeruginosa, there was an increase in pH compared to the control. - After irradiation treatment and inoculation with L. casei, there was a decrease in pH compared to the control. |
Kim et al. (2004) |
| Pseudomonas aeruginosa (NA, 37°C, 48 h) Lactobacillus casei (MRS, 37°C, 48 h) |
|||
| Pork (minced pork, bologna sausage) | Electron beam (2.5 MeV, 2, 4, 6, 8, 10 kGy) X-Ray (5 MeV, 2, 4, 6, 8, 10 kGy) |
- Compared to electron beams, X-rays showed lower protein solubility, detection of total anaerobic bacteria, CIE L*, and CIE a*, but higher TBARS, CIE b*, and cooking yield. - Treatment with 2 kGy or more resulted in increased collagen solubility, TBARS, CIE a*, and CIE b*, and decreased detection of total anaerobic bacteria and CIE L*. - In fresh pork, treatment with 2–6 kGy using electron beams reduced the detection of total anaerobic bacteria by 1.69–2.88 Log CFU/g, and no detection was observed from 8 kGy onwards. - In fresh pork, treatment with 2–6 kGy using X-rays reduced the detection of total anaerobic bacteria by 1.99–2.94 Log CFU/g, and no detection was observed from 8 kGy onwards. |
Shin et al. (2014) |
| Total microorganisms (PCA, 37°C, 48 h) | |||
| Beef (ground beef) Antioxidants (cinnamaldehyde, ascorbic acid, inorganic pyrophosphate) |
Gamma rays (2 kGy) | - Treatment with 2 kGy resulted in a reduction of mesophilic bacteria, psychrotrophic bacteria, and yeasts and molds by 2.58, 3.76, and 1.32 Log CFU/g, respectively. - Treatment with 2 kGy showed no significant difference in pH and general components, but there was a decrease in heme iron content and an increase in TBARS, peroxide, CIE L*, and CIE a*. |
Ayari et al. (2016) |
| Mesophilic bacteria (PCA, 30°C, 3 day) Total coliforms (VRBA, 30°C, 24 h) Isolated Escherichia coli (VRBA, 44°C, 24 h) Yeast and mold (SD, 25°C, 3–5 day) Staphylococcus aureus (MSA, 35°C, 24–48 h) Listeria monocytogenes (PALCAM, 35°C, 24–48 h) Bacillus cereus (EE, 30°C, 24 h) |
|||
| Storage conditions 0, 7, 12, 21 days |
|||
| Herring (canned) | Gamma rays (1.5, 3, 6 kGy) Electromagnetic radiation, X-rays (4.8 MeV, 1.5, 3, 6 kGy) |
- Gamma irradiation and electron beam treatment at 6 kGy resulted in the detection of microbes at approximately 1 Log CFU/g. - X-ray treatment at 3–6 kGy resulted in detection of microbes at approximately 1 Log CFU/g. - Treatment at 6 kGy resulted in the generation of a ‘smoking’ aroma. |
Sanzharova et al. (2021) |
| Mesophilic bacteria Conditions Anaerobes (PCA, 32°C) Salmonella spp. (Bismuth sulphite agar) Total coliforms (MacConkey agar) Listeria spp. (Oxford agar) Staphylococci spp. (Chapman Stone agar) |
|||
| Storage conditions 15, 30 days |
The inclusion of guava leaf extract, which contains antioxidants, has shown to inhibit aerobic microorganisms and E. coli even at a dose of 2 kGy, indicating that natural antioxidants maintain their activity post-radiation (Sadiq et al., 2023). Furthermore, synthetic antioxidants such as inorganic pyrophosphate, cinnamaldehyde, and ascorbic acid have demonstrated significant inhibitory effects against mesophilic and thermophilic microorganisms, with antibacterial activity ranking in the order of inorganic pyrophosphate, cinnamaldehyde, and ascorbic acid (Ayari et al., 2016).
Additionally, antioxidants like tea polyphenols, grape seed extract, D-sodium erythorbate, chitosan, carvacrol, and mangosteen pulp have proven effective in reducing microbial presence during radiation (Chen et al., 2023; Fitrianto et al., 2024; Hu et al., 2021). It is suggested that incorporating these antioxidants could reduce the necessary radiation dose for microorganisms with high radiation resistance or for finished products that require the prevention of external contamination factors during consumption.
Harmful microorganisms that form resistant spores or are highly resistant to radiation require treatment at higher doses than common harmful microorganisms (Lv et al., 2021). Notably, Bacillus cereus, Proteus mirabilis, and Enterococcus faecalis exhibit D10-values for gamma rays and electron beams that are approximately twice as high as those for general harmful microorganisms such as E. coli and Staphylococcus spp. This differential resistance should be taken into account when selecting the appropriate disinfection radiation dose (De Lara et al., 2002; Ebrahim et al., 2022). Moreover, a radiation dose of 45 kGy has been found adequate for sterilizing Clostridium spp. in various meat products, including smoked turkey, chicken fillet, chicken luncheon, beef luncheon, ground meat, and raw sausage (Ebrahim et al., 2022).
It is recommended that radiation sterilization treatment of pet food raw ingredients should be conducted within the maximum allowable dose of 10 kGy. Furthermore, quality and sensory properties studies should be performed for each type of raw ingredient to ensure safety and acceptability. For finished pet food products, it is necessary to conduct quality and sensory properties studies to determine the appropriate radiation sterilization dose, particularly for microorganisms with high contamination potential, considering the shape and distribution form of the product, such as pouches or cans.
Conclusion
The commercialization of non-thermal sterilization technology in the food industry is actively underway, and introducing non-thermal sterilization for pet food is considered highly valuable as the culture of pet humanization spreads. The main non-thermal sterilization technologies are HPP, plasma, and radiation. Unlike traditional sterilization methods such as chemical and heat sterilization, these non-thermal methods offer quality and sensory advantages, including leaving no residue on food, minimizing deformation of raw ingredients, and preserving nutrients.
Autoclaving involves the application of high-pressure to raw ingredients or finished products, ensuring even sterilization across the entire surface of the sample, regardless of its shape, nature, or packaging status. However, protein gel formation and color changes can occur at pressures of 200–400 MPa, making it crucial to identify pressures that effectively sterilize microbes at lower pressures. Therefore, it is necessary to study the sterilization efficiency and quality parameters such as color, water retention, shear force, and sensory properties like flavor and panel evaluation between raw and finished pet food products.
Plasma technology applies plasma sterilization to sterilize the surface of raw ingredients or finished products. Dielectric barrier plasma technology, which uses dielectrics to expand the usable range and lower the treatment temperature, is primarily studied. However, dielectric barrier plasma is less economical than PAW. Additionally, PAW can impart a sour taste depending on the type of treated water, necessitating a comparison of the efficiency of both methods. Consequently, studying the quality and sensory properties of pet food according to various plasma methods is essential.
Radiation involves penetrating raw ingredients or finished products with radiation and can be conducted while the sample is packaged. However, certain radiation doses may cause discoloration and off-flavors, requiring pet sensory studies. Therefore, selecting the appropriate radiation dose and conducting quality and sensory properties studies for various ingredients and finished products is crucial in the radiation treatment of pet food.