Introduction
Religious perception and consumer behavior are closely intertwined, particularly in the context of food selection (Essoo and Dibb, 2004). Each religion has its own unique set of regulations and dietary guidelines. Within the context of Islam, there are certain foods that are permitted to be consumed, known as halal, and others that are prohibited, known as haram (American Halal Foundation, 2023). These days, owing to the exponential growth in the Muslim population worldwide, there has been a noticeable increase in the demand for halal food. Global market research predicts this increase will reach a compound annual growth rate (CAGR) of 6.1% by 2027 (Shafaki, 2023). This is particularly important for halal meat and meat products, as they serve as a valuable protein source and account for 30% of the total demand when combined with poultry and seafood (IMARC, 2023).
Nevertheless, meeting the high demand for halal meat and meat products is not as simple as it may seem. Within the halal food categories, the process of preparing halal meat and meat products adheres to the most strict guidelines, as specified in the holy Quran and Hadith (Quran, 6:118–119; 16:115; Hadith No.17 of Imam Nawawi by Sahih Muslim). Despite the strict requirements, halal meat and meat products are easily accessible to Muslim consumers. They can be readily found in butcher markets, supermarkets, grocery stores, and online stores, all with clearly visible halal labels (Nakyinsige et al., 2012). Over many years, this halal label has been sufficient to instill Muslim consumers’ confidence in the adherence to halal standards in meat or meat products (Nakyinsige et al., 2012). However, there has been a recent and noticeable increase in cases of “illegal meat.” This includes meat that does not comply with halal standards or has been obtained through illegal means (McElwee et al., 2017).
Instances of non-compliance mainly pertain to improper halal slaughtering techniques, mislabeling, or the presence of prohibited materials due to adulteration and contamination (Fuseini et al., 2017). Here are several alarming cases reported in the halal meat and meat products sector. One case that stands out is the 2013 Irish “beef” scandal, wherein beef burgers were found to contain horse DNA and pork (O’Mahony, 2013). A different case is the 2017 halal certification fraud in Brazil, where some large meatpacking companies engaged in unlawful conduct, resulting in the exportation of expired or contaminated halal meat (Silvestre et al., 2018). In addition, in 2018, a well-known halal food brand in the UK encountered controversy when it was revealed that certain products contained non-halal ingredients (Lever, 2020). These varied illegal meat cases ignited a heightened awareness among Muslim consumers or halal enthusiasts regarding the significance of halal authenticity (Fuseini et al., 2017). Serious measures must be taken to protect consumers and restore trust in halal certification.
On the other hand, the concept of halal encompasses more than just the meat or meat product itself. It covers every step of the supply chain, from slaughtering to meat and meat product processing, packaging, labeling, storage, distribution, and retailing. Every step has its own potential areas of non-compliance (Fig. 1). Vulnerabilities in the assessment of halal standards at any stage of the supply chain could be exploited by individuals seeking personal gain (Fuseini et al., 2017). Thus, it becomes imperative for halal bodies to conduct more systematic and comprehensive analyses of halal evaluations and monitoring procedures to ensure the integrity of halal products throughout the supply chain. Relying solely on physical examinations, documentation, and sharia expertise may not provide a comprehensive assessment (Ng et al., 2022).
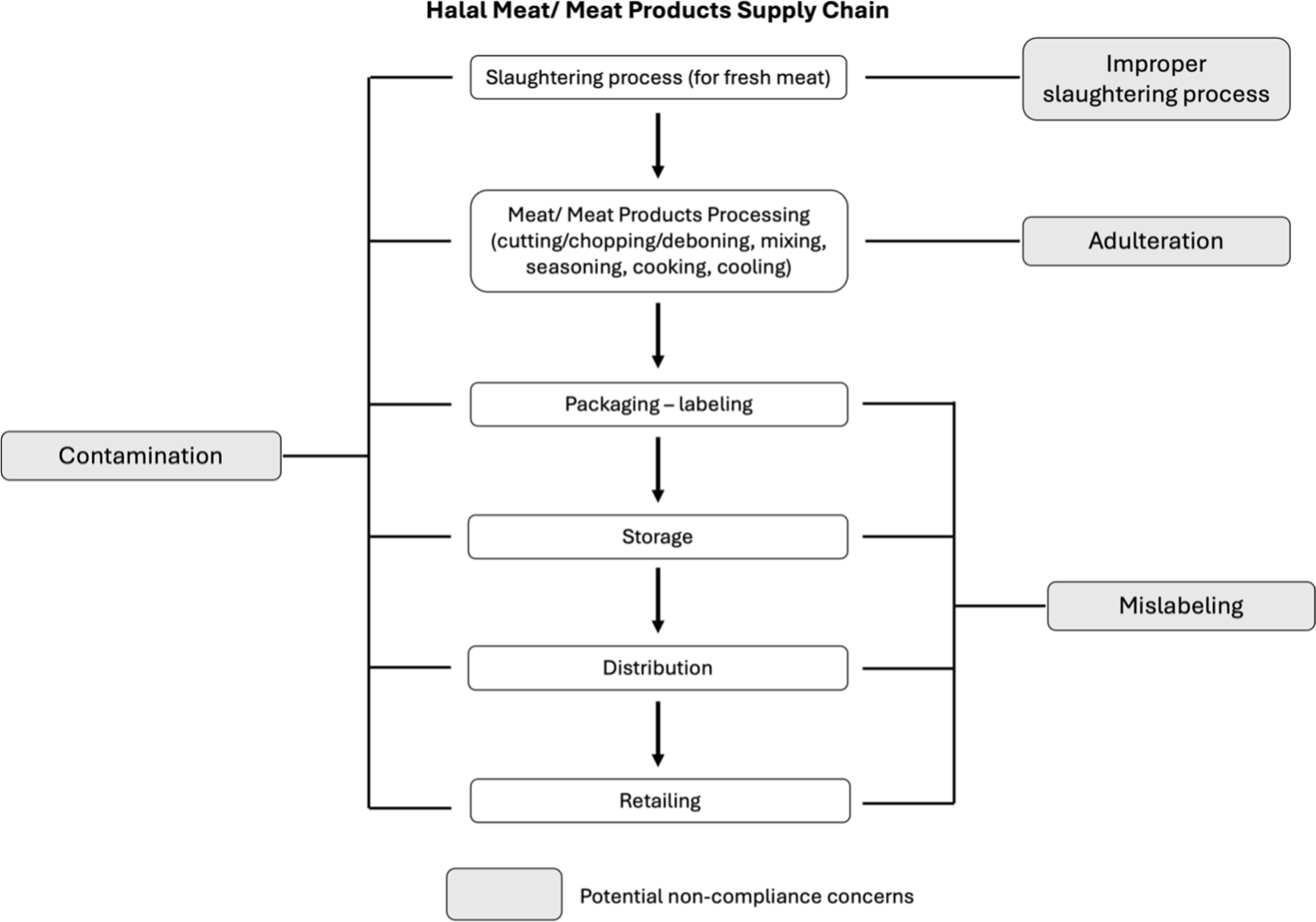
Recent advancements in food science and technology have greatly influenced the progression of halal meat and meat product authentication methods. Researchers have actively developed analytical instruments to address non-compliance concerns in various meats and meat products. Despite previous reviews that have tracked methodological advancements, there remains a gap in connecting these advancements with practical perspectives. Existing reviews primarily focused on advances in various approaches and the categorization of methods based on the use of biological samples. These reviews often divide the discussion into three main categories: DNA-based, protein-based, or spectroscopic-based approaches (Hossain et al., 2020; Ng et al., 2022). While these types of reviews are valuable for understanding method development and tracking analytical progress, they may overlook the overall objectives and concerns regarding non-compliance that each study aims to address. As a result, there is a risk of redundancy and overlap among many studies. Therefore, this review seeks to fill this gap by categorizing analytical methods based on their practical objectives, with a specific focus on research that tackles issues of non-compliance related to the authenticity of halal meat and meat products. These issues encompass improper slaughtering, mislabeling, adulteration, and contamination. In this perspective, our aim is not only to identify existing research gaps and emphasize areas requiring further development but also to provide viable suggestions for enhancing future halal authentication research strategically.
Literature Review
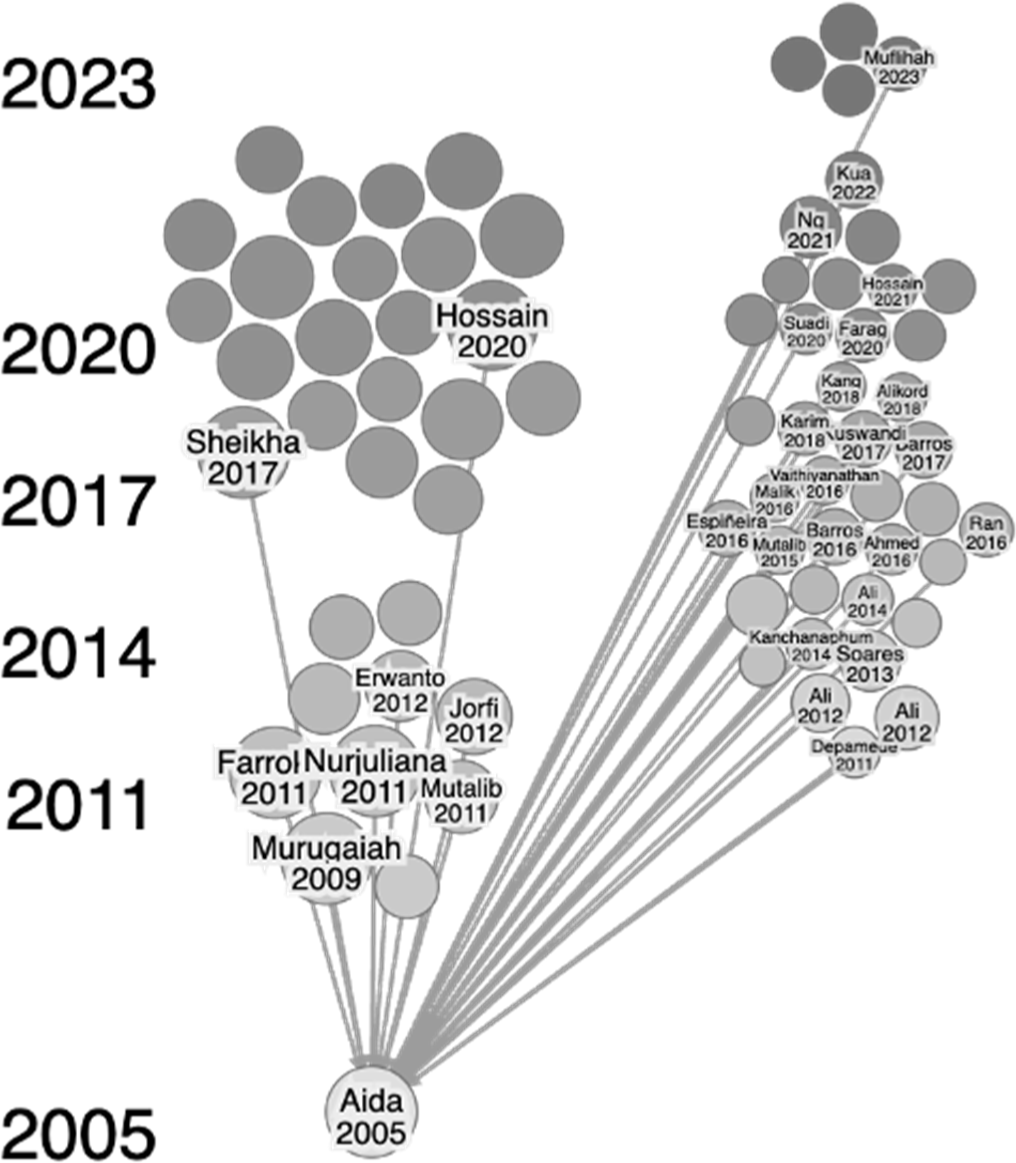
The present review article provides an in-depth exploration of the research conducted on halal authentication methods for meat and meat products using the citation-based literature mapping tool: Research Rabbit (Cole and Boutet, 2023). Three keywords were entered: halal, meat, and authentication. A total of 50 papers, including various original articles, reviews, and proceedings, were selected. These papers were illustrated with dots on the left side and served as the basis for researching other interconnected papers, identified by dots on the right side. It is important to remember that the connection between the articles is based on citation, meaning that some articles on the right side may not have a direct correlation to the authenticity of halal meat. Consequently, we further employed a meticulous selection process to include only papers directly relevant to the topic.
Through the careful organization of the papers in chronological order, it became apparent that the pioneering research on authenticating halal meat was carried out by Aida et al. (2005; Fig. 2). We next limited our literature search from Aida’s study (Aida et al., 2005) to the most recently published articles (2023) to ensure that advances in methodologies and technology remained relevant. Simultaneously, the research was divided into four groups depending on their objectives or potential to address noncompliance issues: improper slaughtering, mislabeling, adulteration, and contamination. In the sections that follow, we carefully review each category separately.
Main Issue
Halal and non-halal slaughter methods differ significantly in their procedures and underlying principles. Halal slaughter adheres to Islamic dietary guidelines, including the invocation of Allah’s name, a specific method of cutting the animal’s throat, and strict animal welfare standards (American Halal Foundation, 2023). In contrast, non-halal slaughter lacks these religious and ethical standards. The rigorous halal standards have unfortunately led some deceitful individuals to bypass these standards, resulting in an increase in the sale of meat that does not comply with halal slaughtering requirements but is falsely labeled halal (Fuseini et al., 2017). This highlights the importance of reliable halal authentication methods to maintain consumer trust and uphold religious dietary guidelines.
The halal checking process in slaughterhouses is usually conducted by well-trained experts who meticulously assess halal compliance. They thoroughly evaluate various aspects, such as the pre-slaughtering process, the knife used, the person in charge, the invocation made, and the method of slaughtering (American Halal Foundation, 2023). Although relying on trained experts for halal evaluation has proven effective, this approach comes with inherent limitations, including the potential for inaccuracies and the subjective nature of the process (Bonne and Verbeke, 2008). To address these challenges, the incorporation of analytical instruments is deemed necessary (Ng et al., 2022).
We explored relevant articles with a specific emphasis on the procedure of halal slaughtering. Our review indicated that there is still a lack of studies on identifying halal slaughtered and non-halal slaughtered meat (Table 1). The most recent study, conducted in 2023 by Bouzraa and colleagues, evaluated the quality of beef meat produced using halal, halal with stunning, and non-halal slaughter techniques. The quality was evaluated by measuring the amount of microorganisms (aerobic mesophilic bacteria, enterobacteria, and coliforms) and biomarkers related to animal welfare (glucose, cortisol, lactate dehydrogenase, and creatine kinase; Table 1). The study’s results showed that these two parameters can effectively differentiate the quality profile of each type of meat based on the technique of slaughter. Specifically, the halal with stunning technique produced meat with minimal microbial counts and high animal welfare biomarkers, while the non-halal slaughter technique produced contrasting results (Bouzraa et al., 2023).
| Non-compliance concern | Meat/meat product | Method/technology | Summary of findings | Reference |
|---|---|---|---|---|
| Improper slaughtering | Beef | Microbial analysis and physiological parameters | - Microbiological counts vary (p<0.05) based on the type of slaughter (regular, halal, halal with stunning), indicating differences in meat hygiene. - The type of slaughter affects (p<0.05) physiological parameters in blood samples, including glucose, lactate dehydrogenase, creatine kinase, and cortisol. |
Bouzraa et al. (2023) |
| Lamb | Instrumental and sensory analyses | - Meat quality assessments were conducted on two muscles: M. longissimus thoracis et lumborum and M. triceps brachii. - Slaughter following electric head-only stunning (EHOS) and post-cut electric head-only stun (PCEHOS) techniques resulted in quicker muscle discoloration compared to traditional halal slaughter without stunning (TNS). - No significant differences in sensory attributes between the three methods. |
Danso et al. (2017) |
Additionally, there is another study that aimed to evaluate the quality of halal lamb by comparing two halal slaughter techniques: stunning and non-stunning, using instrumental and sensory analysis (Danso et al., 2017; Table 1). Instrumental analysis revealed that lamb muscles slaughtered using the halal stunning technique had a faster discoloration rate than those slaughtered using the halal non-stunning technique. Whereas, the sensory score for both halal slaughtered techniques was found to be comparable. These results demonstrated that instrumental analysis had the potential to identify differences in halal lamb meat quality across different slaughtering techniques. However, further research is necessary to determine the actual effectiveness of this analysis in comparing halal and non-halal slaughtering techniques.
The two studies discussed above have shown promising results in improving halal evaluation in slaughter processes. However, more research is needed to continue advancing this field. The available literature on halal slaughtering of poultry products may provide valuable insights that can assist in the development of methods to evaluate halal meat slaughtering. Researchers have measured the levels of hemoglobin in the muscles from halal and non-halal slaughtered rabbits (Nakyinsige et al., 2014), analyzed the levels of biogenic amines in halal and non-halal slaughtered chickens (Yusoff et al., 2021), and examined the chicken’s esophagus using image processing and artificial intelligence (AI; Yusof et al., 2020). Implementing these research strategies may help in the acceleration of research efforts and hence enhance the reliability of the halal slaughtering evaluation process.
Furthermore, halal authentication involves not only verifying that the process of production complies with regulations. It also ensures that the label information accurately matches the description of the materials or components used (Chuah et al., 2016). This is critical; even halal authorities have suggested that the labels on the packaging should provide all the necessary information for consumers. This includes the factory name, meat type, product weight, ingredients list, production date, expiry date, handling instructions, and a guarantee from the factory that the product meets quality standards and is correctly labeled according to consumer standards and importing country requirements (American Halal Foundation, 2023).
However, in recent times, there has been a rise in reported cases of halal meat and meat products mislabeling, which can be intentional or unintentional (Fuseini et al., 2017). The intentional cases were mostly driven by monetary benefits. Often involving adulteration practices where permissible components were mixed with more affordable forbidden (haram) ones (Chuah et al., 2016). On the other hand, unintentional cases were frequently caused by contamination from instruments, equipment, or careless handling along the supply chain (Supian, 2018). Regardless of the underlying motivation, it is important to develop methods for checking the correctness of labels in relation to their contents. The primary focus of this section would be on research aimed at developing methods for label verification. Meanwhile, in the following section, we will delve deeper into studies relating specifically to issues of adulteration and contamination.
According to our review of the literature, there was a limited amount of research on the development of label verification for halal meat and meat products. Current available methods covered the use of DNA-based methods and computational technology (Table 2). Multiplex polymerase chain reaction (PCR) was used in a study to validate halal labeling in pre-packaged beef and poultry meat products (Chuah et al., 2016). The result of this study found that only 21.7% of processed meat products had accurate labeling, with the vast majority of the products being mislabeled. This suggests that the developed analytical technique represents a promising strategy for verifying halal labeling.
| Non-compliance concern | Meat/meat product | Method/technology | Summary of findings | Reference |
|---|---|---|---|---|
| Mislabelling | Prepacked meat products (beef and poultry) include sausages, cold-cut meats, cooked whole muscle meats, breaded products, meatballs, and ground meats. Sausages, cold-cut meats, cooked whole muscle meats, breaded products, meatballs, and ground meats. | Multiplex polymerase chain reaction (PCR) | - Utilized species-specific primers for meat species identification. - Identified a high mislabeling rate of 78.3% in the samples. |
Chuah et al. (2016) |
| Packaged food | Optical Character Recognition (OCR) technology | - OCR technology employed for character recognition on halal product packaging. - Front-end system utilized mobile device camera. - Communication with back-end system facilitated through web service technology. - Application successfully identified halal products based on label information. |
Yuniarti et al. (2017) | |
| Packaged food | Deep learning technology: convolutional neural networks (CNNs) | - CNNs employed for non-halal composition detection in packaged foods via image processing. - Identification of non-halal compositions involved combining characters into words and comparing with a list. - Segmentation process significantly influenced accuracy, resulting in 50% overall word accuracy. - Main error linked to incorrect segmentation. |
Fadhilah et al. (2018) |
Furthermore, researchers have also created applications to detect mislabeling. One such application, Latext (Halal Text), utilized the integration of optical character recognition with internet of things technologies (Yuniarti et al., 2017; Table 2). The application captured the text shown on the package, specifically the E-number, which represents codes for food additives, and validated its correctness with a web service-connected backend system. The trial of this smartphone Latext application resulted in the ability to properly check label data by integrating information from a web-based service. Another separate study used Convolutional Neural Networks (CNN) models to identify non-halal content on halal food product packaging labels (Fadhilah et al., 2018). CNN was commonly utilized for the recognition of handwritten numeric images. The image of the label was segmented into individual characters and classified using CNN. The characters were subsequently converted into text format and compared with an identification list of non-halal raw materials. The system achieved a character recognition accuracy of 98.08% but only 50% accuracy for character verification against the existing list.
The aforementioned analytical method and computational technologies had the potential to effectively address concerns related to mislabeling in the halal meat and meat products industry, which was quite appealing. In addition, there are other DNA-based methods, like DNA barcoding and random amplification of polymorphic DNA fingerprints (Arslan et al., 2005), that could be used as alternative analytical methods to confirm halal meat labeling. Thus, the examination of these approaches for use in halal meat and meat products, along with the possibility of incorporating computational technology, offers a novel strategy that deserves more consideration and experimentation.
The occurrence of mislabeling issues was frequently linked to adulteration, which refers to the deliberate mixing or substitution of permitted materials with prohibited ones (Mortas et al., 2022). This issue is particularly concerning in the context of halal meat and meat products. Numerous studies have explored different methods and instruments to identify adulteration (Mortas et al., 2022), with PCR- and chromatography-based methods emerging as the most popular and extensively studied (Table 3). Table 3 lists a range of methods employed in identifying adulteration, along with a summary of the findings.
| Non-compliance concern | Meat/meat products | Analytical method/technology | Summary of findings | Reference | |
|---|---|---|---|---|---|
| Adulteration | Detection of rat meat in beef meatball | Molecular spectroscopy-based methods | Fourier transform infrared spectroscopy (FTIR) | - Spectral data from 3,100–800 cm–1 used for analysis. - Beef and rat meatballs differentiated using linear discriminant analysis. - Lipid composition differences revealed by FTIR spectra. |
Lestari et al. (2022) |
| Identification of chicken, chevon, beef and donkey meat | Nuclear magnetic resonance (NMR) | - Identified 37 metabolites in cow, goat, donkey, and chicken muscle using 1H-NMR. - Lactate, creatine, and 10 other metabolites distinguished white (chicken) from red meat (chevon, beef, donkey). - Inosine, uracil, carnosine, and 3 others differentiated chevon, beef, and donkey. |
Akhtar et al. (2021) | ||
| Detection of Pork in beef sausages | Near-infrared spectroscopy (NIR) | - Three methods for multivariate analysis were established: laboratory, fiber optic probe, and on-site. - Laboratory and fiber optic setups detected meat and fat adulteration down to 10%. - On-site setup detected meat adulteration effectively and fat adulteration up to 20% (quartz cuvettes) or 40% (polymer packaging). |
Schmutzler et al. (2015) | ||
| Identification of pork fat with other fats | Fluorecents light spectroscopy | - The developed method could effectively distinguish between pure pork, a mixture of pork, and samples without any pork based on the analyzed spectrum patterns. | Islam et al. (2021) | ||
| Detection of rat and wild boar meat in beef meat | Chormatography-based methods | Gas chormatography (GC) | Annotated potential metabolites marker: - Beef class: dimethylfulvene - Rat class: benzyl alcohol - Wild boar class: 1,3,5-cycloheptatriene - Mixture of beef and rat class: benzaldehyde, 3-ethyl - Mixture of beef and wild boar class: 2,6-dimethyldecane |
Amalia et al. (2022) | |
| Detection of horse and pork in highly processed food | High performance liquid chromatography (HPLC) | - Identified stable marker peptides for thermal processing of meat products. - Enabled to detecti of pork or horse at low concentrations (0.24% concentration) in beef matrix. - Developed a rapid 2-minute extraction protocol for protein extraction from processed food. |
von Bargen et al. (2014) | ||
| Detection of pork in Pangasius hypopthalmus meat (PHM) | Liquid chromatography (LC) | - Authentic and adulterated PHM were reliably distinguished (R>0.95 and Q>0.5). - Identified PC(o-18:0/18:2(9Z,12Z)) as a potential metabolite marker and dimyristoylphosphatidylcholine as a potential marker for PHM. - Myoglobin and β-hemoglobin peptides were identified as pork indicators. |
Windarsih et al. (2022) | ||
| Identification of pork, beef, and chicken | - A chemometrics-assisted shotgun proteomics approach using PCA and orthogonal partial least squares discriminant analysis (OPLS-DA) was employed to identify peptide markers. - Glu-C endoproteinase was used for peptide identification. - Peptide specificity was validated through in vitro analysis. |
Yuswan et al. (2018) | |||
| Identification of chicken, beef, and pork sausages | Polymerase chain reaction (PCR)-based methods | Simplex and multiplex-PCR | - Cytochrome Oxidase SubUnit I primers were effective in identifying bovine, porcine, and chicken DNA in sausages with a high sensitivity of 0.001 ng/μL. | Boyrusbianto et al. (2023) | |
| Detection of dog, pork, and rat meat in beef meatball | Simplex-, duplex-, and multiplex-PCR | - Multiplex-PCR with 12S rRNA gene primers could detect bovine, dog, pig, and rat species in beef meatballs in one reaction. | Cahyadi et al. (2020) | ||
| Identification of pig meat and fat from other animals | PCR-RFLP (restriction fragment length polymorphisms) | - The cyt b PCR-RFLP species identification assay exhibited excellent results for detecting pig meat and fat. | Aida et al. (2005) | ||
| Detection of pork in processed meat products | - The assay was able to detect 0.0001 ng of swine DNA in pure formats and 0.01% (w/w) spiked pork in extensively processed ternary mixtures of pork, beef, and wheat flour. | Ali et al. (2011) | |||
| Pork adulterated in raw and cooked sausages | PCR-QIAxcel capillary electrophoresis | - PCR-QIA procedure efficiently differentiated targeted DNA fragments, even at low levels (0.01% pork/meat: w/w). | Barakat et al. (2014) | ||
| Detection of dog meat in beef meatball | Real time-PCR | - Real-time PCR using Cyt b-55 primer detected dog meat DNA at concentrations as low as 0.25 ng/mL, equivalent to 1% of dog meat in beef meatballs. | Manalu et al. (2019) | ||
| Identification of pork DNA in meat (beef and chicken) extracts | SYBR green I-real-time PCR | - The assay was able to achieve a low detection limit of 0.1 ng of porcine DNA. | Farrokhi and Jafari Joozani (2011) | ||
| Detection of wild boar meat in beef meatball | Species-specific PCR | - The q-PCR assay with CYTBWB2-wb primers successfully detected wild boar meat DNA at low concentrations of 5 pg/μL. | Aina et al. (2019) | ||
| Identification of cat, dog, pork, monkey, and rat meat | - The assay detected 0.01–0.02 ng of DNA from raw dog, pig, monkey, and rat meats and 1% of probable meatball constituents. | Ali et al. (2015) | |||
| Detection of pork meat in beef, mutton, and chicken | qPCR (quantitative PCR) | - The assay showed high sensitivity and a low detection limit of 2.7 ng/μL for total DNA from pork meat. | Wu et al. (2021) | ||
| Identification of porcine in meat products | qPCR and doplet digital PCR (ddPCR) | - QPCR and ddPCR exhibited comparable linearity (r2=0.9971 and 0.9998, respectively). - While detection limits were similar, ddPCR demonstrated superior sensitivity at low DNA concentrations. |
Nuraeni et al. (2023) | ||
| Identification of pork in raw beef, and chicken meat, and a mixture of processed meat | Nanotechnology | Gold nanoparticles (GNPs) | - Developed an electrochemical DNA biosensor using GNP-DNA probe bioconjugates on SPCE-Gold. - Optimized biosensor using 40 μL of 153 μg/mL bioconjugates, 20-minute immobilization, and 60-minute hybridization. |
Hartati et al. (2019) | |
| Identification of beef, pork, rabbit, and chicken meat profile and meat powder | Differential scanning calorimetry (DSC)- | - DSC was used to verify the halal status of beef and its byproducts. - The results showed an endothermic peak for each. |
Nugrahani and Aditya (2023) | ||
| Detection of pork in beef floss | Immunoassays-based methods | Enzyme-linked immunosorbent assay (ELISA) | - ELISA was more effective than conventional PCR for intensely heated product samples. - Processed meat products might contain inhibitory chemicals that can affect species identification. |
Aprilia et al. (2022) | |
| Detection of pork in meat extract | Molecularly imprinted polymer nanogels (MIP-NGs) | - Developed a rapid PSA detection system using nanogels and antibodies. - Analysis time under 30 minutes. - Effective in detecting 0.01 wt% pork adulteration in halal meat. |
Cheubong et al. (2023) | ||
| Identification of pork meat and pork sausages from beef, mutton, and chicken meats and sausages | Electronic nose | - Combining electronic nose technology, GCMS-HS analysis, and PCA for halal verification purposes gave the samples a good separation with 67% of the total variance. | Nurjuliana et al. (2011) | ||
| Identification of beef and pork meat | - The classification results showed a high accuracy of 98.10% in detecting beef and pork using the optimized support vector machine. | Sarno et al. (2020) | |||
A substantial portion of research has focused on the identification and quantification of pork in halal meat or meat products using various PCR assays. Ranging from the most basic assay, singleplex PCR, to more sophisticated assays like multiplex PCR, real-time PCR, PCR-RFLP (restriction fragment length polymorphisms), PCR-QIAxcel capillary electrophoresis, SYBR green I-real-time PCR, species-specific PCR, qPCR, and ddPCR (Table 3). This comprehensive array of PCR assays indeed showcased the versatility of PCR in offering diverse tools for discerning and quantifying the presence of pork. However, to enhance the development of PCR-based methods, future research efforts should move beyond assay diversity.
One notable limitation of DNA-based analysis lies in the potential for cross-reactivity with closely related species or conserved regions in non-target organisms. This inherent limitation significantly elevates the risk of false positive results, particularly when discerning between halal and non-halal meat from the same permissible animal species. Addressing this limitation requires comprehensive exploration, delving into intricate samples, and optimizing assays to enhance specificity. In addition, research on identifying prohibited animals beyond pork remains limited. While successful detection methods have been established for wild boar, rats, and dogs (Aina et al., 2019; Ali et al., 2013; Cahyadi et al., 2020), more comprehensive studies are needed. Such comprehensive analysis would contribute to the development of robust PCR methods for authenticating halal meat and meat products, leading to more reliable results.
Furthermore, we also explored the trend in the chromatography-based method category, encompassing methods such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and liquid chromatography (LC) coupled with mass spectrometry (MS; Table 3). Chromatography-based methods focus on analyzing metabolites extracted from the sample matrix, specifically meat and meat products in this context. Each chromatographic instrument possesses a specific range of metabolite coverage. For example, GC analysis is commonly used to identify markers within volatile compounds. One study successfully identified specific volatile compounds that can be used to differentiate between beef, rat, wild boar, and their mixtures. These compounds, such as dimethylfulvene and benzyl alcohol, serve as unique chemical fingerprints for each meat type (Amalia et al., 2022). Conversely, HPLC and LC are frequently used to explore metabolite markers within peptides, lipids, and larger molecular weight groups. For instance, HPLC has demonstrated the ability to identify specific peptides that can be used as markers to detect very low levels of pork or horse meat in beef products, as low as 0.24% (von Bargen et al., 2014). Additionally, LC-HRMS has identified specific lipid molecules, such as PC(o-18:0/18:2(9Z,12Z)) and DMPC, as potential markers for differentiating meat types (Windarsih et al., 2022).
While these methods show promise, identifying the precise origins of these markers remains a challenge. Considering that the measurement was conducted on the final products that have completed the entire supply chain process, it is plausible that these markers may originate from the meat production process rather than the animal’s metabolism or distinctive meat traits (Trivedi et al., 2016). This could introduce inconsistencies and inaccuracies. As such, we suggested that future research efforts should approach this complexity cautiously, perform further validation, and acknowledge the possibility of confounding factors. Additionally, researchers are encouraged to include detailed information about the limitations of the study, which can serve as valuable guidance for future investigations.
Although PCR and chromatography-based methods are frequently employed, they may not be the most convenient alternatives. The need for faster and more practical detection methods has led to the development of biosensors and electronic noses (e-noses; Raja Nhari et al., 2023; Table 3). While biosensors and e-noses share a common goal of detecting and analyzing specific compounds, they differ fundamentally in their technologies. Biosensors use biological components like enzymes, antibodies, or nucleic acids to convert signals into measurable outputs. For instance, one notable study was conducted by Cheubong et al. (2023). In this study, molecularly imprinted polymer nanogels (MIP-NGs) were used as detectors, complemented by antibody detection methods. The MIP-NGs biosensor technologies exhibited a remarkable sensitivity and delivered rapid analysis results. It was able to detect pork adulteration in halal beef and lamb meat, with a detection limit of 0.01 wt%, within a timeframe of less than 30 min (Cheubong et al., 2023). On the other hand, e-noses, designed to emulate the human olfactory system, utilize sensor arrays to identify volatile compounds present in the air. In a recent study by Sarno et al. (2020), the Optimized Electronic Nose System was introduced. This system achieved an impressive accuracy rate of 98.10% within 15 min, demonstrating the potential of e-nose technology for rapid and accurate differentiation of meat types and products.
Although biosensors and e-noses show promise in detecting meat adulteration, significant advancements are required to improve their sensitivity and accuracy. The complex nature of meat samples, combined with various processing techniques and storage conditions, complicates the differentiation of closely related samples. To address these challenges, a comprehensive approach integrating multiple analytical methods is required. By combining highly sensitive techniques like PCR and chromatography with biosensors and e-noses, a robust reference database can be created. Furthermore, leveraging AI in this system can significantly enhance the accuracy and practicality of detecting adulteration in halal meat and meat products.
Furthermore, our review revealed a notable intersection in the research on detecting both adulteration and contamination. Both areas of study shared a common objective: detecting the presence of prohibited materials, such as blood, pork, and pork derivatives (Supian, 2018). The key distinction only lies in the intent behind these occurrences—adulteration tends to be intentional, while contamination is typically unintentional (Fuseini et al., 2017). In light of this, we argued that the research outlined in the adulteration section (Table 3) could effectively contribute to detecting contamination as well. Despite this alignment, we recognized the importance of further exploring literature that specifically aimed to address the problem of contamination. This would offer additional insights into the nuanced landscape of contamination detection. In this pursuit, we identified several studies that met above-specified criteria. Table 4 summarizes these studies, which involve the utilization of various methods such as densitometry analysis, high-resolution melting analysis (HRMA; Denyingyhot et al., 2021), monoclonal antibodies (MAbs; Raja Nhari et al., 2016), MIP-NG-based sensors (Cheubong et al., 2021), and interdigitated electrodes (IDE; Nordin et al., 2016).
| Non-compliance concern | Meat/meat product | Method/technology | Summary of findings | Reference |
|---|---|---|---|---|
| Contamination | Pork contamination in halal beef and goat sausages | Densitometry analysis | - Actin fraction <50 kDa) identified as a potential biomarker for detecting pork in processed meat products. - Precision and accuracy tests (KV<5%, percent recovery>95%) confirmed the method’s effectiveness in testing halalness, particularly for pork-contaminated sausages. |
Hermanto et al. (2022) |
| Six prohibited meats (donkey, cat, pig, rat, dog, and monkey) contamination in halal beef meatballs and other commercial food products | High resolution melting analysis (HRMA) | - Prohibited animal DNA limit of detection: 0.01 ng (except pig DNA, which is 0.001 ng). - Method achieved 100% accuracy in identifying intentionally adulterated non-halal meats in beef meatballs. - Method validation with 260 Thai food products identified two samples contaminated with pig DNA. |
Denyingyhot et al. (2021) | |
| Porcine blood contamination | Monoclonal antibodies (MAbs) | - Qualitative ELISA characterized MAbs against blood, non-blood, and plasma from different species. - Twelve MAbs exhibited specificity to porcine plasma. - MAbs recognizing 60 kDa heat-treated soluble proteins in porcine blood and plasma were selected as a novel approach for detecting porcine plasma in processed food. |
Raja Nhari et al. (2023) | |
| Pork contamination in beef extract | Molecularly imprinted polymer nanogel (MIP-NG)-based sensor | - Fluorescent molecularly imprinted polymer nanogel (F-MIP-NG) sensor exhibited excellent analytical performance to detect porcine serum albumin. - Rapid detection, less than 5 minutes per sample. - Low detection limit of 0.1 wt% for pork contamination. |
Cheubong et al. (2021) | |
| Porcine contamination | Interdigitated electrode (IDE) | - Titanium dioxide (TiO2) deposition on IDEs for optimization. - IDE could detect porcine presence at 1.0 μM. - Gold replacement may enhance device sensitivity. |
Nordin et al. (2016) |
Upon a thorough examination of these studies (Table 4), certain discernible patterns emerged. First, there was a common focus in all the studies, which revolved around the development of methods to detect the presence of pork, whether in samples of halal meat or meat products. With the exception of the study employing HRMA, a method was developed not only to detect pork but also to identify other prohibited animals, including donkeys, cats, rats, dogs, and monkeys. Second, the variability across all studies is notable in the choice of biological materials employed for analysis. Densitometry studies utilized protein extracts, while HRMA and IDE procedures were reliant on DNA. On the other hand, MAbs utilized plasma material, contrasting with MIP-NG-based sensors that utilized serum material. Collectively, these studies have shown encouraging findings and added to our knowledge of the various methods used to detect pork contamination in halal meat and meat products. This diverse range of detection options enables halal certification bodies and other stakeholders to select methods that best suit their specific requirements and analytical capabilities.
However, despite the progress in analytical methods, it is still quite difficult to ensure the complete absence of contamination throughout the supply chain. The challenge lies in the need to trace and identify contamination sources, requiring testing at all crucial points along the supply chain. In order to achieve this, it is necessary to have a resilient method that can adapt to a variety of settings and environments. Therefore, we suggest focusing future research efforts on enhancing the durability of current methods. This strategic approach has the potential to strengthen the reliability of halal evaluation in meat and meat products, ultimately contributing to the mitigation of contamination occurrences.
According to our review results, it is evident that most of the studies of halal meat and meat product authentication were centered around methods for detecting adulteration and contamination. Meanwhile, there have been limited studies conducted on the evaluation of slaughtering techniques and labeling accuracy. In light of this research trend, we suggest that future developments in methods for detecting adulteration and contamination should shift towards refining the practicality of existing analytical methods. Recent developments in biosensors and e-noses have demonstrated encouraging progress in the field of practical methods, providing valuable insights for further exploration.
Prioritizing practicality, in our perspective, can result in the creation of tools that are more efficient and accessible. This, in turn, may lead to higher adoption rates among halal bodies, potentially reducing certification costs and thereby lessening the financial burden for producers. As acceptance grows, iterative development may begin to take place, enabling the opportunity to learn from previous versions, identify weaknesses, and make necessary improvements. This dynamic approach has the potential to further enhance the effectiveness of detecting adulteration and contamination, particularly in more intricate samples or challenging conditions.
On the other hand, when it comes to less-explored areas of research like detecting improper slaughtering techniques and mislabeling, diversifying analytical instruments and improving accuracy and sensitivity are more essential. This will ensure that halal bodies and producers have a broader range of alternatives for assessing these non-compliance concerns. It is also important to note that being able to detect prohibited materials in final halal meat and meat products may not reveal information about processing practices or ingredient sources. Therefore, ensuring the accuracy of the slaughtering process and labeling is of utmost importance.
Moreover, variations in halal regulations regarding both aspects have heightened the importance of advancement in this area. Though a global halal standard is available, certain regions have made adjustments to align with local customs and traditions (Akbar et al., 2023). For instance, Australia and New Zealand permit stunning prior to slaughter and mechanical slaughter (Nakyinsige et al., 2014), whereas other countries advocate for traditional hand slaughtering without stunning (Akbar et al., 2023; Nakyinsige et al., 2014). Aside from that, there are also variations regarding the permissibility of certain ingredients (Akbar et al., 2023). Unfortunately, these varied viewpoints and details are often not explicitly disclosed on packaging labels. Consequently, the varying regulations, coupled with the lack of clear information, present a significant challenge for consumers seeking to make informed halal choices.
Given these complicated facts, we argue that not only detection methods must be strengthened to address supply chain concerns but also transparency. Currently, labels provide essential information such as halal signs, product details, and quality standards declarations. However, a gap exists in providing results of halal evaluation and monitoring (Bonne and Verbeke, 2008). To ensure halal integrity and promote transparency, it is imperative to integrate advanced analytical methods and technology into halal evaluation while making the resulting data easily accessible. Blockchain technology can be used to achieve this transparency by providing a secure and open way for participants to store and share data (Abidin and Perdana, 2020). The decentralized nature of blockchain assures that information is irreversible and dependable. Moreover, network participants must verify the accuracy of information when adding new blocks, ensuring that all members can access the same data (Abidin and Perdana, 2020). This holistic solution strengthens halal evaluation, bridges the information gap, and reinforces trust among stakeholders in the halal meat supply chain. Most importantly, openly sharing detailed halal information with consumers empowers them to make well-informed choices, instilling confidence in the safety of the halal meat products they purchase.
Summary
The concerns surrounding the halal meat and meat product supply chain, including improper slaughtering techniques, mislabeling, adulteration, and contamination, pose a threat to the authenticity of halal certification. Consequently, we assessed the gap in halal authentication research to propose suggestions for enhancing halal evaluation and assisting consumers in verifying halal claims. Based on our review, significant progress has been made in identifying adulterants and contaminants; however, a gap persists in developing accessible and user-friendly analytical tools. Simultaneously, advancing research on slaughterhouse practices and label integrity is crucial for maintaining comprehensive halal standards. Furthermore, the integration of cutting-edge technologies such as biosensors, e-noses, and blockchain offers groundbreaking potential for supply chain oversight and assessment. By prioritizing practicality, precision, and transparency, we can build a resilient and reliable halal meat supply chain that meets the growing demands of the global Muslim consumer base.













