Introduction
Animal blood is a common byproduct in meat production, often found in slaughterhouses (Fallows and Wheelock, 1982). In 2023, Thailand produced around 9.6 million bovines (Office of Agricultural Economics, Thailand), with an estimated 15-liter blood recovery rate per bovine Kastner et al. (2012), totaling 144 million liters. Bovine blood consists of 35%–40% cellular components and 65%–70% plasma, containing water (80.9%), protein (17.3%), fat (0.23%), carbohydrates (0.07%), and minerals (0.62%; Duarte et al., 1999). Slaughterhouses sell blood at affordable prices for human food, animal feed, and fertilizer. However, managing the substantial blood volumes and environmental pollution from direct disposal remain challenges with limited research attention.
Concerns arise regarding the biological safety of blood from slaughtered animals, necessitating heat treatment to eliminate potential pathogens before its use in human or animal food (Göhl et al., 1982). Native blood proteins, like fibrinogen, are resistant to digestion, making heat treatment essential to enhance protein digestibility (Tanka-Salamon et al., 2010). Finding new applications for blood components is a challenge, but extracting bioactive compounds offers economic opportunities and innovation in slaughterhouse blood utilization. Researchers are investigating various uses, including bioactive peptide production. Bioactive peptides, short chains of amino acids, offer beneficial effects on biological systems (Korhonen and Pihlanto, 2006), with potential applications in food science and technology as ingredients, additives, or amino acid sources (Mardani et al., 2023). Enzymatic production of peptides is preferred for its mild conditions, resulting in stable and reproducible products (Nasri, 2017). Various enzymes like alcalase, flavourzyme, and trypsin are explored for generating antioxidative peptides (Nasri, 2017), which exhibit diverse biological activities such as antioxidant, angiotensin-converting enzyme (ACE) inhibitory, dipeptidyl peptidase-IV (DPP-IV) inhibitory, antimicrobial, and lipid-lowering effects (Kim and Wijesekara, 2010; Sangsawad et al., 2022; Suwanangul et al., 2021), depending on their amino acid composition and sequence. Research on bioactive peptides from animal blood has focused on separating blood into red blood cells and plasma, yielding antioxidant peptides, particularly from bovine blood (Aung et al., 2023; Bah et al., 2016; Lafarga et al., 2016). Traditional methods involve using anticoagulants, resulting in elevated sodium levels in the final product and limiting its use. Our study intentionally used bovine blood without anticoagulants to address this issue.
Excessive reactive oxygen species (ROS), triggered by external factors or diseases, can cause oxidative stress, harming normal cells (Davalli et al., 2016). Oxidative stress is linked to diseases like hypertension, diabetes, and cancer (Li et al., 2019; Yang and Lian, 2020), emphasizing the importance of antioxidants in disease prevention and treatment. Synthetic antioxidants have limitations due to side effects, prompting interest in natural compounds like antioxidant peptides found in various sources, including milk, meat, and plants (Mardani et al., 2023). These peptides, typically 2–20 amino acids long, exhibit antioxidant properties like scavenging radicals and chelating metals (Shahidi and Zhong, 2008).
According to enzymatic processing, a mixture of peptides can be further utilized through concentration or separation into distinct fractions based on molecular sizes, employing an ultrafiltration (UF) membrane method. Notably, antioxidant peptides originating from whole bovine blood remain unidentified and uncharacterized. The discovery of bioactive peptides holds the potential to provide valuable insights into the relationship between protein structure and function. Additionally, computer simulation (in silico) proves instrumental in replicating the gastrointestinal (GI) digestion of peptide sequences, offering insights into the structural alterations of bioactive peptides. This simulation aids in designing targeted hydrolysis processes and ensuring the preservation of a given peptide’s sequence during GI digestion (Parastouei et al., 2023). Moreover, conducting an in vivo study comes with substantial costs. Thus, before moving on to the in vivo testing phase, validating the bioactive peptide through in silico digestion is essential.
Consequently, the objectives of this study encompass the inaugural production of antioxidant peptides from whole bovine blood without the use of an anticoagulant, along with the concentration via UF, characterization, and identification of the active peptide sequence. Furthermore, the study seeks to predict the structural changes of antioxidant peptides through in-silico GI digestion.
Materials and Methods
Various chemicals and reagents for the experiments were procured from Sigma-Aldrich (St. Louis, MO, USA) including 2,4,6-trinitrobenzene-1-sulfonic acid, ethylenediaminetetraacetic acid (EDTA), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), Trolox, trinitrobenzene sulphonic acid (TNBS), 2,4,6-tripyridyl-s-triazine (TPTZ), ferrozine, acetonitrile (ACN), trifluoroacetic acid (TFA), Cytochrome-C, aprotinin, and tyrosine. Alcalase® 2.4 L FG (2.4 AU-A/g) was purchased from Novozymes (Bagsvaerd, Denmark). Neutrase (0.8 AU-N/g) was also procured from Brenntag Ingredients (Bangkok, Thailand). Papain (6,000 USP U/mg) was from Solarbio (Beijing, China). GL Biochem (Shanghai, China) supplied synthetic peptides with a purity level exceeding 98%. All other chemicals and reagents utilized were of analytical grade.
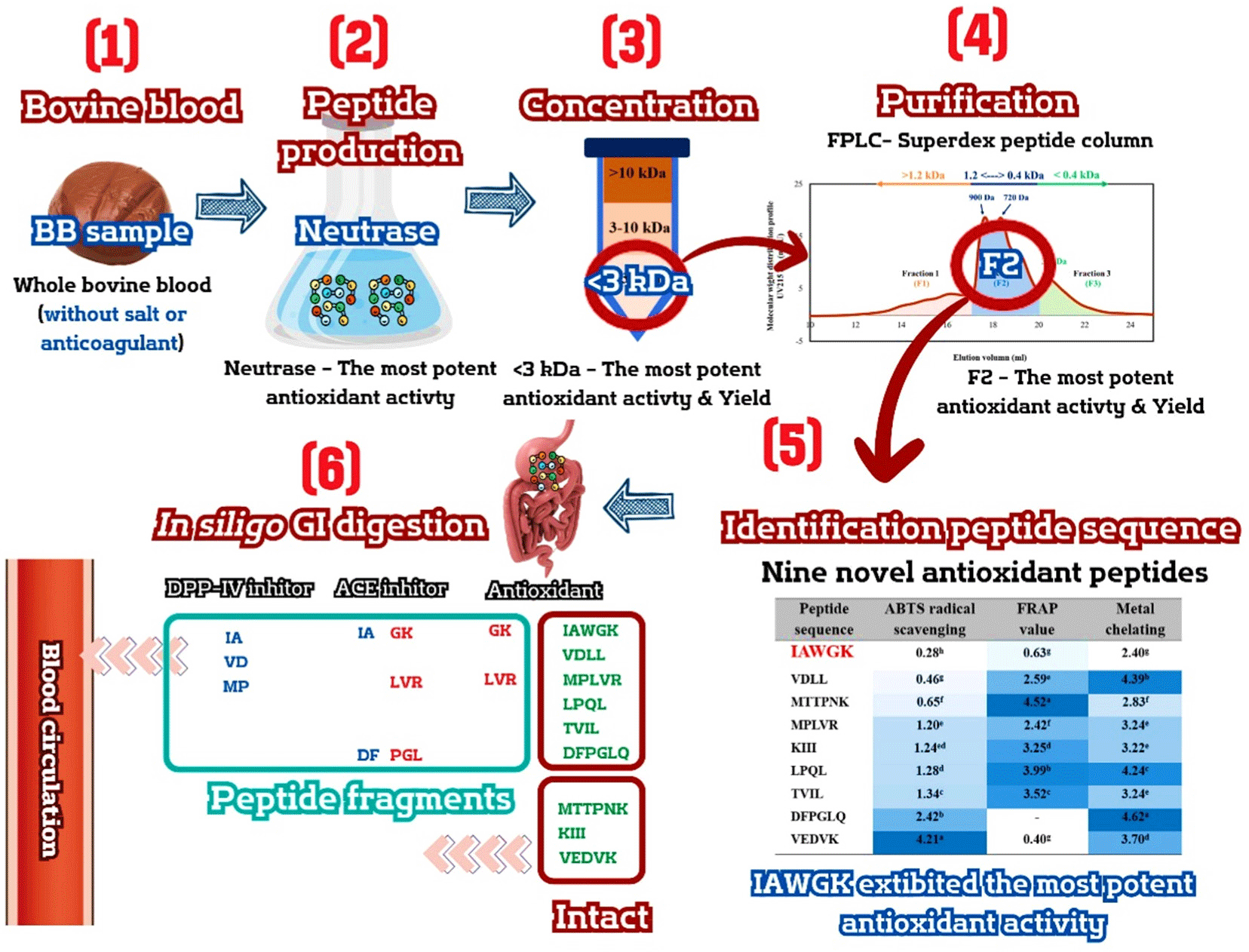
Fresh blood samples of approximately 500 mL were collected from each bovine (20 bovines aged 30–36 months) using sterile containers immediately after slaughter. These bovines were crossbred Thai native Brahman Charolais without the use of sodium chloride and sodium citrate salts as an anticlotting agent. After collecting the blood, the samples were pooled and intentionally boiled at 90°C for 30 min. The specific cooking condition was employed to achieve protein denaturation and eliminate pathogenic organisms. This process was conducted and supplied by the Pon-Yang-Khram Livestock Breeding Cooperative NSC (Sakon Nakhon, Thailand). Following this, it was carefully cooled on ice until the core temperature reached 4°C, after which it was transferred to a freezer set at –20°C until analysis. Before analysis, the frozen blood sample, which had been boiled previously, was thawed and finely ground using a grinder. It was then labeled as “BB.” Subsequently, it was randomized for proximate analysis. This analysis encompassed determining crude protein via the Kjeldahl method, assessing crude fat through Soxhlet extraction, and evaluating moisture and ash content according to AOAC recommendations (Latimer, 2016). The summary diagram illustrating the sample preparation process is displayed in Fig. 1.
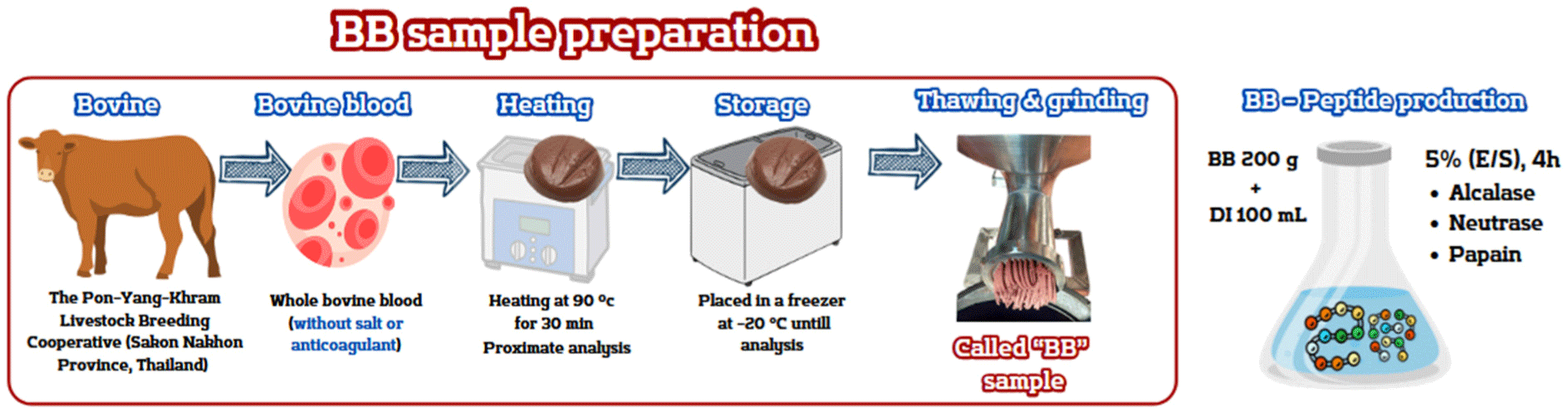
The 200 g bovine blood (BB) sample was randomized sampling, comprising 32.5 g of protein, and was blended with 100 mL of deionized (DI) water in four replications. Following this, alcalase, neutrase, and papain, commercial enzymes, were employed for hydrolysis, each at a 5% concentration (enzyme/protein substrate, w/w), exhibiting specific enzyme activities of 2.4 AU-A/g, 0.8 AU-N/g, and 6,000 USP U/mg, respectively. Furthermore, distinct hydrolysis conditions were maintained for each enzyme: alcalase was operated at a pH of 9.6 and a temperature of 50°C, neutrase at a pH of 7.0 and a temperature of 50°C, and papain at a pH of 7.0 and a temperature of 65°C. The mixture was shaken at 120 rpm in a water bath shaker for 4 h, with pH adjustments being executed every 2 h using 1 M NaOH or HCl. The enzyme reaction was terminated by heating at 95°C for 30 min. Subsequently, the final pH was adjusted to 7.0, and the supernatant was obtained following centrifugation at 10,000×g for 10 min at 4°C. The supernatants are being held at –20°C until further analysis. Aliquots of samples were retained separately for protein recovery, degree of hydrolysis (DH), antioxidant activity, molecular weight distribution, and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), as described in the following sections.
Protein recovery indicates the proportion of the protein content successfully harvested in the supernatant after hydrolysis. The supernatant was collected and examined for the amount of protein content using the Kjeldahl method following the AOAC (Latimer, 2016), compared to the total protein content of the original sample. The protein recovery was calculated as follows:
In assessing peptide content peptide content containing α-amino groups, we employed the TNBS method, following procedures outlined by Khongla et al. (2023). The peptide sample (10 μL) was mixed with 100 μL of 0.2125 M phosphate buffer (pH 8.2) and 50 μL of 0.05% TNBS solution in a 96-well microplate. L-leucine was selected as the representative α-amino acid. Using a microplate reader (Varioskan LUX, Thermo Fisher Scientific, Vantaa, Finland), we measured absorbance at 420 nm at 45°C for 30 min. The DH signifies the proportion of cleaved peptide bonds, which was determined using the TNBS method. The calculation involves α-amino groups and is expressed as:
A and B represent α-amino acid contents after 0 and 4 h of hydrolysis, respectively. T represents the total α-amino acid, achieved through 100% hydrolysis in 6 N HCl at 120°C for 24 h.
The ABTS assay was conducted following the method outlined by Suwanangul et al. (2022). In a 96-well microplate, a 5 μL peptide sample (1 mg/mL of peptide concentration) was combined with a fresh 200 μL ABTS solution. The reaction was allowed to incubate in darkness for 5 min before being measured at 734 nm using a microplate reader (Varioskan LUX, Thermo Fisher Scientific). For result calculation, a trolox standard curve ranging from 0 to 2.5 mg/mL was employed, and the activity was expressed in terms of μg/mL trolox equivalence.
The ferric reducing antioxidant power (FRAP) assay was conducted following the method outlined by Suwanangul et al. (2021). In a 96-well microplate, a 10 μL peptide sample (1 mg/mL of peptide concentration) was combined with a fresh 100 μL FRAP reagent. The reaction was allowed to incubate at room temperature for 15 min before being measured at 593 nm using a microplate reader (Varioskan LUX, Thermo Fisher Scientific). For result calculation, a trolox standard curve ranging from 0 to 2.5 mg/mL was employed, and the activity was expressed in terms of μg/mL trolox equivalence.
The metal chelating activity assay was conducted following the method outlined by Sangsawad et al. (2016). In a 96-well microplate, a 10 μL peptide sample (1 mg/mL of peptide concentration) was mixed with 100 μL of DI, 10 μL of 2 mM FeCl2, and 10 μL of 5 mM ferrozine. The reaction was incubated at dark room temperature for 20 min before being measured at 562 nm using a microplate reader (Varioskan LUX, Thermo Fisher Scientific). For result calculation, an EDTA standard curve ranging from 0 to 1 mg/mL was employed, and the activity was revealed in terms of μg/mL EDTA equivalence.
Molecular weight distribution analysis is essential for understanding peptide profiles. It allows us to explore molecular patterns, compare different molecular weight profiles, display the percentage of each peptide size, and estimate the DH. The approach outlined by Sangsawad et al. (2022) was implemented in assessing the distribution of molecular weights. The analysis of the peptide profile was executed through the utilization of size exclusion chromatography (SEC). The peptide sample (100 μL, with a concentration of 7 mg/mL) underwent SEC utilizing a Superdex Peptide 10/300 GL column (GE Healthcare, Piscataway, NJ, USA). Elution (30 mL) was performed in an isocratic mode at a flow rate of 0.7 mL/min, employing a mixture of 30% ACN and 0.1% TFA in DI water. Measurement of UV215 nm absorption was employed to ascertain the peptide profile. The standard molecule used for determining molecular weight comprised cytochrome-c, aprotinin, synthetic peptides, and tyrosine.
UF was employed to fractionate the peptide derived from neutrase hydrolysis (BB-N), distinguishing them by their molecular weight and concentration through the selective removal of larger or smaller molecules. This concentration step is vital for downstream applications that require higher concentrations of bioactive peptides. The BB-N peptide was subjected to separation via a UF membrane (Amicon; EMD Millipore, Billerica, MA, USA). Initially, the supernatant (10 mL) of BB-N peptides was channeled through a primary membrane with a molecular weight cutoff of 10 kDa, followed by two rounds of centrifugation with DI water at 5,000×g and 4°C. This process yielded the retentate, containing peptides exceeding 10 kDa in size. Subsequently, the permeate was directed through a secondary membrane with a molecular weight cutoff of 3 kDa, acquiring both permeate and retentate. The former comprised peptides smaller than 3 kDa, while the latter encompassed peptides within the 3–10 kDa range. Determining protein recovery and evaluating antioxidant activities (ABTS radical scavenging, FRAP, and metal chelating activity) were then carried out for each fraction obtained through the UF process.
The protein composition of the samples was analyzed using SDS-PAGE, following the procedure outlined by Luasiri et al. (2024). In this analysis, 1 g of the sample was homogenized in 5% SDS (9 mL) and underwent a 10-min heat treatment at 95°C. After centrifugation at 10,000×g for 10 min, the resulting supernatant was mixed with the sample buffer in a 1:1 ratio. The samples consisted of raw bovine blood, cooked bovine blood (BB), the residue of BB-N hydrolysis, and BB-N peptide in the soluble part. Subsequently, 15 μg of protein was loaded onto 10% acrylamide gels for electrophoresis, maintaining a constant voltage of 120 V for 60 min to facilitate protein separation. The gel was stained with solution A (0.125% Coomassie Brilliant Blue R-250) for 120 min and subjected to destaining with solution B (25% ethanol and 10% acetic acid) for 60 min. This entire process was repeated twice to ensure the accuracy of the results.
The peptide obtained from the permeate of UF-3 kDa, which showed the highest antioxidant activity and peptide yield, was purified using SEC, following Khongla et al. (2022) procedure. A 300 μL of peptide sample, with a concentration of 20 mg/mL, was subjected to the SEC system, where a Superdex Peptide 10/300 GL column (GE Healthcare) was employed. Elution (30 mL) was performed in an isocratic mode at a flow rate of 0.4 mL/min, utilizing DI water. UV215 nm absorption measurements were employed for the analysis of the peptide profile. The resulting peptide fraction was collected, dried, and evaluated for both peptide yield and antioxidant activity. Peptide content determination was conducted using the TNBS method, following the procedures outlined by Adler-Nissen (1979). The calculation of peptide yield was carried out as follows:
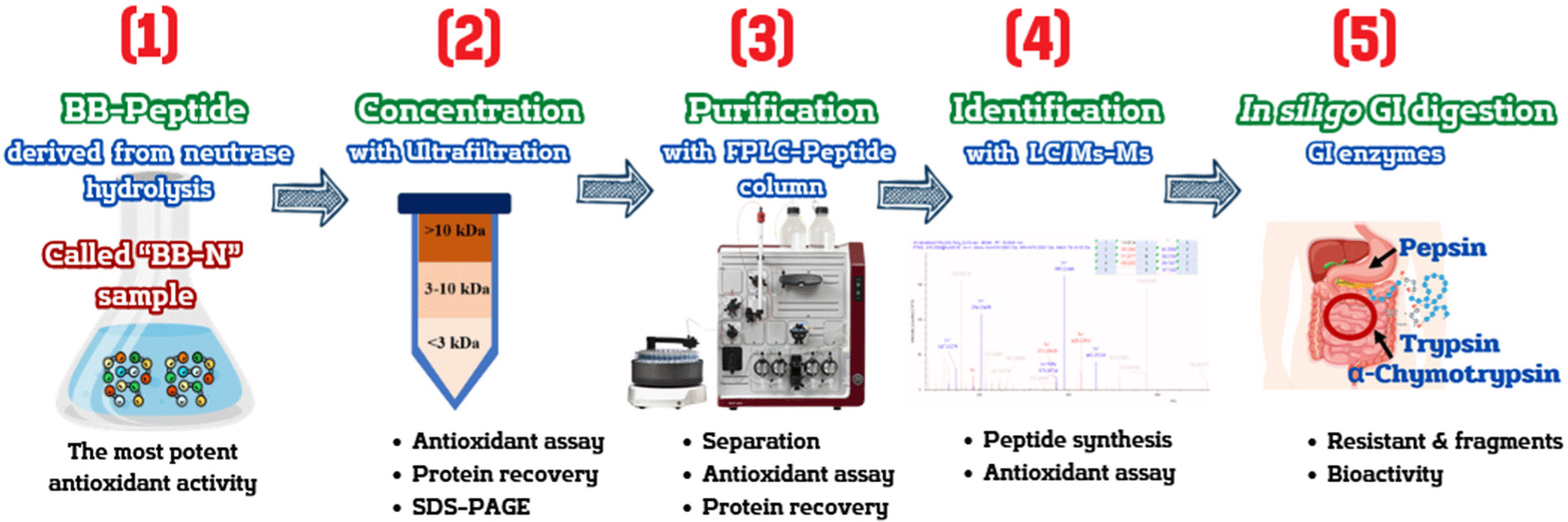
Following the procedure outlined by Sangsawad et al. (2018b), identifying the peptide sequence was performed on the F2-fraction with molecular weight 0.4–1.2 kDa derived from SEC, which exhibited the highest antioxidant activity and peptide yield. The peptide was cleaned up by Peptide desalting spin columns (Thermo Fisher Scientific) and determined using LC-HRMS. The analysis was performed on Dionex Ultimate 3000RS LC system interfaced with Q Exactive Plus high-resolution mass spectrometer with an ESI source (Thermo Fisher Scientific). A 2 uL of peptides (75 ng) was injected onto an easy-spray pepmap C18 column (15 cm×75 μm with a particle size of 3 μm, Thermo Fisher Scientific) at 35°C with a column flow rate of 0.3 mL/min. The mobile phases consisted of 0.1% formic acid in aqueous (mobile phase A) and 0.1% formic acid in ACN (mobile phase B) using gradient elution program as follows: 0–5 min, 3% B, 5–50 min, 20% B, 50–55 min, 30% B, 55–60 min, 80%B; (holding until at 61 min), 61–65 min, 3% B; (holding until at 75 min). For mass spectrometry analysis, ESI source settings had an energy range of 2 kV and S-Lens RF level 60. The capillary temperature was maintained at 320°C. Full scan (resolution 70,000) with data-dependent MS2 (dd-resolution 17,500) scan mode was utilized, and the top 10 precursor ions were first selected for ddMS2. Data acquisition was executed with a scan range of m/z 200–1,600 and normalized collision energy 30 eV. The system was regulated by Xcalibur 4.3 software, and the MS raw files were subjected to data processing via the Proteome Discoverer 2.4 program (Thermo Fisher Scientific) for de novo sequencing. The peptide sequence was identified using bovine blood protein obtained from the UniProt database as the parent protein. The Synchrotron Light Research Institute (Public Organization) undertook the entire process.
The criteria for peptide synthesis necessitated the selection of peptides with “de novo” scores exceeding 70%. The peptide sequences identified in bovine blood were subjected to synthesis using the solid-phase peptide synthesis method. Following synthesis, the antioxidant properties of these peptides were assessed through ABTS radical scavenging, FRAP, and metal-chelating activity assays, with IC50 values serving as expressions. In silico digestion of each peptide was done by utilizing pepsin, trypsin, and chymotrypsin facilitated by the DFBP program (http://www.cqudfbp.net/). The resulting peptide fragments were subsequently analyzed. Moreover, a comprehensive exploration of reported bioactivity for both intact and fragmented peptides was conducted through the DFBP program.
The stages of antioxidant peptide production in the bovine blood sample include production, concentration, purification, identification, and in silico GI digestion. It is visually depicted in Fig. 2, offering a detailed schematic illustration.
The SAS 9.4 software was utilized to execute a one-way analysis of variance in the data. In total, the experiments were designed using a completely randomized design in four replications. The mean and SD of the data were computed. The differences were assessed using Duncan’s multiple-range test (Jones et al., 2001).
Results and Discussion
Our research, focused on producing bioactive peptides, involved a comprehensive examination of a BB sample, resulting in valuable insights into its chemical composition. As illustrated in Table 1, the proximate composition of bovine blood merits attention, with a notable emphasis on its protein content, registering at 16.24% on a wet basis. When recalculated on a dry basis, protein emerges as the predominant component, comprising a substantial 89.33% of the composition. This discovery underscores the considerable potential of bovine blood as a robust source of protein.
| Parameters | Moisture | Protein | Fat | Ash |
|---|---|---|---|---|
| Wet basis | 81.95a | 16.24b | 0.03d | 1.20c |
| Dry basis | - | 89.99a | 0.16c | 6.67b |
To contextualize our findings, we referred to the comprehensive study conducted by Sorapukdee and Narunatsopanon (2017), which extensively outlines the protein compositions across diverse animal blood types. Notably, among these findings, porcine blood stands out with the highest protein content at 90.97%, followed closely by duck blood at 89.70% and chicken blood at 88.27% based on a dry basis, respectively. These comparative insights provide crucial benchmarks, enriching our comprehension of the inherent protein richness in bovine blood. Significantly, this comparative analysis underscores that the protein content of bovine blood falls within the range observed in these alternative animal blood types, thereby emphasizing its substantial protein potential. Consequently, this positions bovine blood as a promising candidate for developing bioactive peptides.
In addition to protein, other components such as moisture, fat, and ash were also considered in our analysis. Lower levels of both fat (0.16%) and ash (6.67%) were observed in bovine blood compared to porcine blood, chicken blood, and duck blood (Sorapukdee and Narunatsopanon, 2017), further contributing to its appeal as a protein source with minimal undesirable components. The findings from Sorapukdee and Narunatsopanon (2017) significantly bolstered our assessment, offering a comprehensive perspective on protein content across various animal blood types. This collaborative insight strengthens the conclusion that bovine blood, distinguished by its high protein and low-fat content, holds considerable promise as an optimal source for producing bioactive peptides.
This study aimed to maximize peptide yield by utilizing a high bovine blood protein substrate content. Initially, we intended to concentrate on the bovine blood sample. However, this approach proved impractical due to the process of collecting blood from the Pon-Yang-Khram Livestock Breeding Cooperative NSC, which lacks the addition of anticoagulants to prevent sodium salt contamination. As a result, the raw blood underwent immediate boiling (90°C for 30 min) to produce blood cake, effectively reducing microbial contamination. Therefore, the boiled bovine blood, without concentration, was utilized as the protein material for this study.
Peptides derived from bovine blood are created by breaking down larger bovine blood proteins into smaller peptide fragments. This process utilizes a range of commercially available proteolytic enzymes, such as alcalase, neutrase, and papain, known for their ability to produce bioactive peptides. The DH is a key factor in this process. The DH is a measure that represents the proportion of peptide bonds in the material that have been enzymatically hydrolyzed. A higher DH indicates that more proteins have been broken down into smaller peptides, increasing free amino acids. The properties of the resulting peptides are influenced by two main factors: the type of enzyme used and the specific conditions under which the hydrolysis process occurs. Different enzymes and conditions can lead to peptides with varying characteristics.
In our research, we tracked the progression of BB hydrolysis by measuring the DH. The results are illustrated in Fig. 3A. Alcalase showed the highest DH among the enzymes used, followed by papain and Neutrase. A higher DH in Alcalase hydrolysis indicates a lower molecular weight of peptides, suggesting that the peptides produced might be smaller than those obtained from papain and neutrase hydrolysis. This elevated DH in alcalase hydrolysis aligns with previous studies like Naveed et al. (2021), which reported that Alcalase, an endopeptidase with broad substrate specificity, cleaves proteins on the carboxyl side of hydrophobic amino acids such as Ala, Val, Leu, Ile, and Met. In contrast, Neutrase, a mild endopeptidase, cleaves peptides like alcalase, targeting the carboxyl side of hydrophobic amino acids. Papain, on the other hand, is a cysteine protease that cleaves on the carboxyl side of aromatic amino acids like Phe, Trp, and Tyr, as well as other amino acids like Leu and Gly (Naveed et al., 2021).
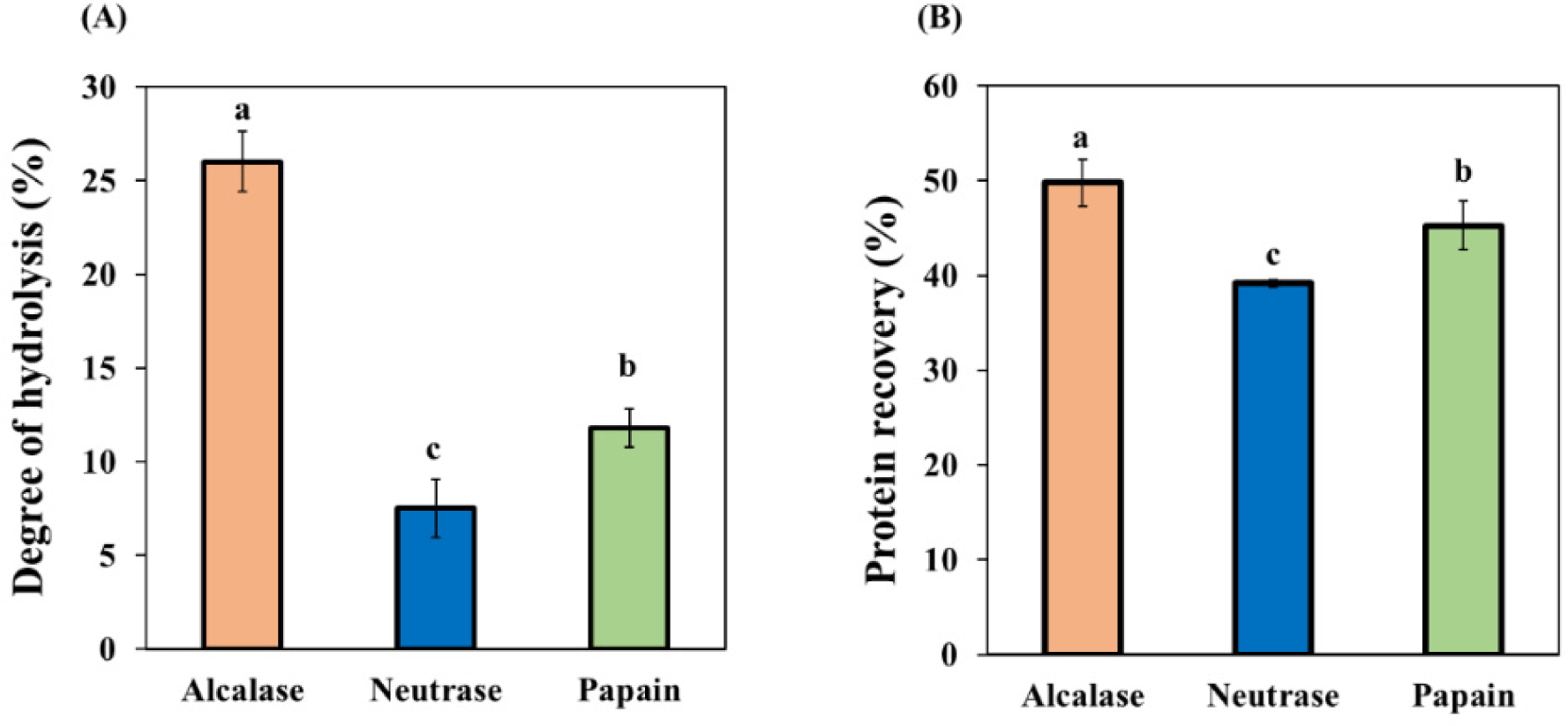
Our findings are consistent with those of Hyun and Shin (2000), who investigated the hydrolysis of bovine blood plasma. They found that the alcalase hydrolysis conditions were the most effective for breaking down blood plasma protein. In addition, Zhou et al. (2012) also found that alkaline proteases, including alcalase, exhibit higher proteolytic activity than neutral proteases like neutrase and papain. The key takeaway from our study is the potential of bovine blood as a source of bioactive peptides, given its high protein content and the effectiveness of alcalase in hydrolyzing these proteins. This result opens new avenues for using bovine blood to produce different sizes of bioactive peptides.
Our results clearly show a correlation between protein recovery and the DH. Alcalase was found to have the highest protein recovery, followed by Papain and Neutrase, as depicted in Fig. 3B. A higher DH suggests an increased yield of peptides. Under the hydrolysis conditions we used, we observed a significant protein recovery rate of 40%–50%. Hyun and Shin (2000) reported protein recovery rates for bovine blood plasma proteins with alcalase, neutrase, and papain at 58%, 16%, and 10%, respectively. Our study demonstrates that all the commercial enzymes tested can hydrolyze BB into highly soluble peptides. The unique cleavage sites of each of the three enzymes result in different peptide products, which could have significant implications. As a result, we also evaluated the antioxidant activities of these peptides in subsequent experiments.
Antioxidant activity is a broad term that describes a peptide’s capacity to inhibit or neutralize the detrimental effects of free radicals or ROS, thereby safeguarding cells and tissues from oxidative damage (Mardani et al., 2023). Peptides possess antioxidant properties attributed to the presence of amino acids that can scavenge free radicals, chelate metal ions, or donate electrons. However, the antioxidant activity of peptides may vary based on the enzyme used and the DH.
In assessing the antioxidant activity of BB peptides using the ABTS technique, as illustrated in Fig. 4A, the antioxidant activity of peptides derived from alcalase, neutrase, and papain hydrolysis showed no significant differences (p>0.05); the results were 354, 356, and 337 μg trolox eq./mL, respectively. This result suggests that bovine blood peptides resulting from these three hydrolysis possess the function of inhibiting ABTS free radicals by donating hydrogen atoms or transferring electrons. This finding indicates that the DH isn’t linked to ABTS radical scavenging. According to Krasae et al. (2023), tuna peptides of varying sizes consistently exhibit ABTS radical scavenging abilities. Moreover, Adebiyi et al. (2009) suggested that peptides containing phenolic hydroxyl group amino acids could contribute to ABTS radical scavenging. Additionally, antioxidative amino acids like His, Pro, Trp, Leu, Val, Ala, and Met likely contribute. Hence, amino acid type and composition may influence BB peptide effectiveness. This result implies that BB peptides from alcalase, neutrase, and papain hydrolysis may have similar amino acid types and compositions.
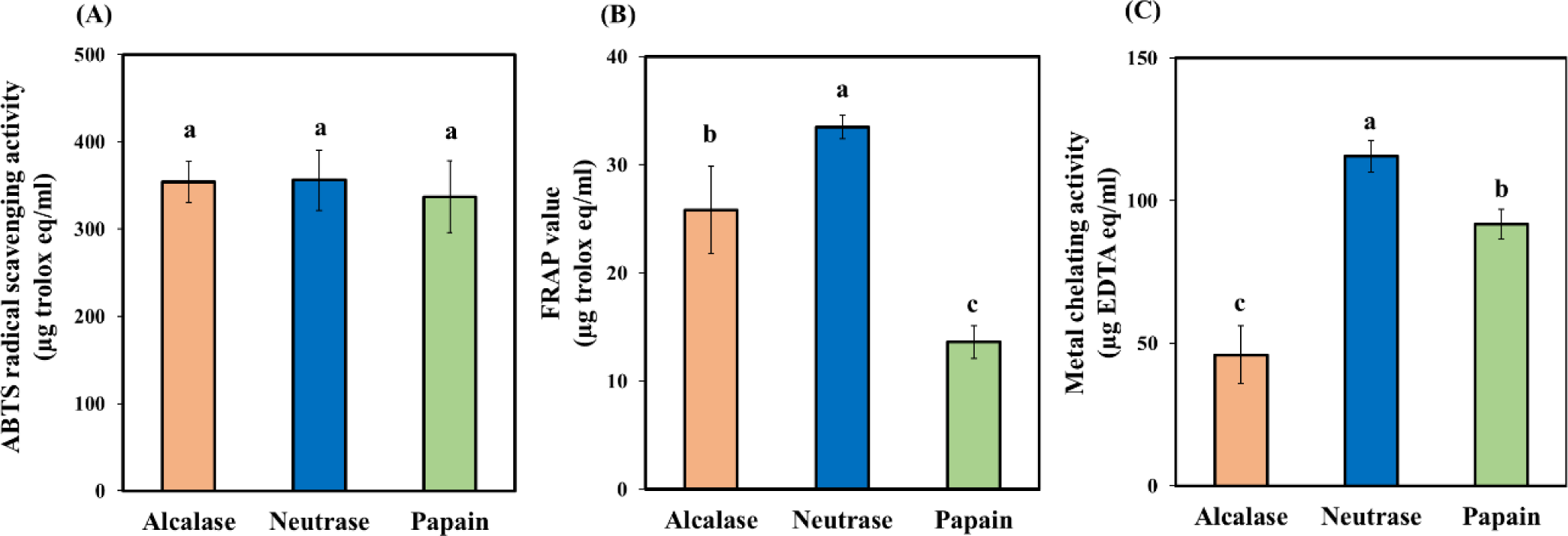
The FRAP method verifies a sample’s antioxidant capacity by its ability to convert Fe3+ to Fe2+ (Cao and Prior, 1998), with the resulting color shift from yellow to blue measured at 593 nm. Notably, the antioxidant activity assessed through the FRAP assay exhibited significant differences (p<0.05), as illustrated in Fig. 4B. The peptide derived from neutrase hydrolysis demonstrated the highest FRAP values, surpassing those from alcalase and papain hydrolysis. The study conducted by Ketnawa et al. (2018) highlighted the effectiveness of peptides abundant in Ala, Leu, Pro, and aromatic amino acids like Trp, Phe, Tyr, and His in combating free radicals through direct electron transfer. This finding suggests that the BB peptide, produced from neutrase hydrolysis, might have the highest concentration of these amino acids, thereby showing enhanced capability in donating electrons to Fe3+.
In assessing antioxidant activity through the metal chelating assay, we measure peptides’ capacity to bind and eliminate metal ions that can generate ROS and induce oxidative stress. The metal chelating assay relies on the interaction between Fe2+ and a colorimetric agent (ferrozine), generating a complex with a specific absorbance (Cao and Prior, 1998). Peptides capable of chelating the metal ion will reduce complex formation, decreasing absorbance. Our research found that peptides derived from neutrase hydrolysis exhibited the highest metal chelating activity, measured at 115.52 μg EDTA/mL, followed by papain and alcalase hydrolysis (Fig. 4C). Our research demonstrates a connection between lower DH values, as illustrated in Fig. 3A, and increased antioxidant activity associated with metal chelation. Although the specific reason for this correlation remains unclear at present, it could be attributed to differences in the amino acid composition and sequence of active peptides present in the samples. Previous studies have shed light on the significance of amino acid sequence in determining antioxidant efficacy. For instance, Egusa Saiga and Nishimura (2013) emphasized the role of terminal amino acid residues’ side chain composition in influencing the metal ion chelation capability of antioxidative peptides. Furthermore, Phongthai et al. (2018) highlighted that the ability of rice bran peptides to chelate metal ions depends not only on peptide size but also on specific structural elements, such as Glu, Asp, His, and Lys residues, which interact with metal ions.
Our findings indicate that the choice of enzyme significantly influences the generation of potent antioxidant peptides. This finding underscores the critical role of enzyme selection in the production of such peptides. Specifically, the BB peptides produced through neutrase hydrolysis exhibited the highest antioxidant activity compared to those generated by alcalase and papain.
The molecular weight distribution of bovine blood peptides, obtained through hydrolysis by alcalase, neutrase, and papain, exhibits distinct profiles, as depicted in Fig. 5A. These profiles range from less than 0.4 kDa to 10 kDa. The elution volume displays prominent peaks at 10 and 25 mL. An analysis of the peptide fractions within these profiles, as shown in Fig. 5B, reveals that peptides within the 0.4–2 kDa size range constitute the majority, accounting for approximately 51%–59%. These peptides are likely tetra- to pentapeptides and typically could be absorbed through epithelial paracellular tight junctions (Hong et al., 2016). Focusing on the peptides derived from neutrase hydrolysis reveals the highest antioxidant activity. The profiles contain the most fractions within the 0.4–2 kDa size range, representing 59.70%, a percentage higher than those obtained from alcalase and papain hydrolysis. Taheri and Bakhshizadeh (2020) reported that medium-chain peptides, with a size of 1–3 kDa, exhibit stronger antioxidant activity than short-chain peptides, which are less than 1 kDa in size. This finding may account for the observed higher activity in the peptides derived from neutrase, which was responsible for 0.4–2 kDa.
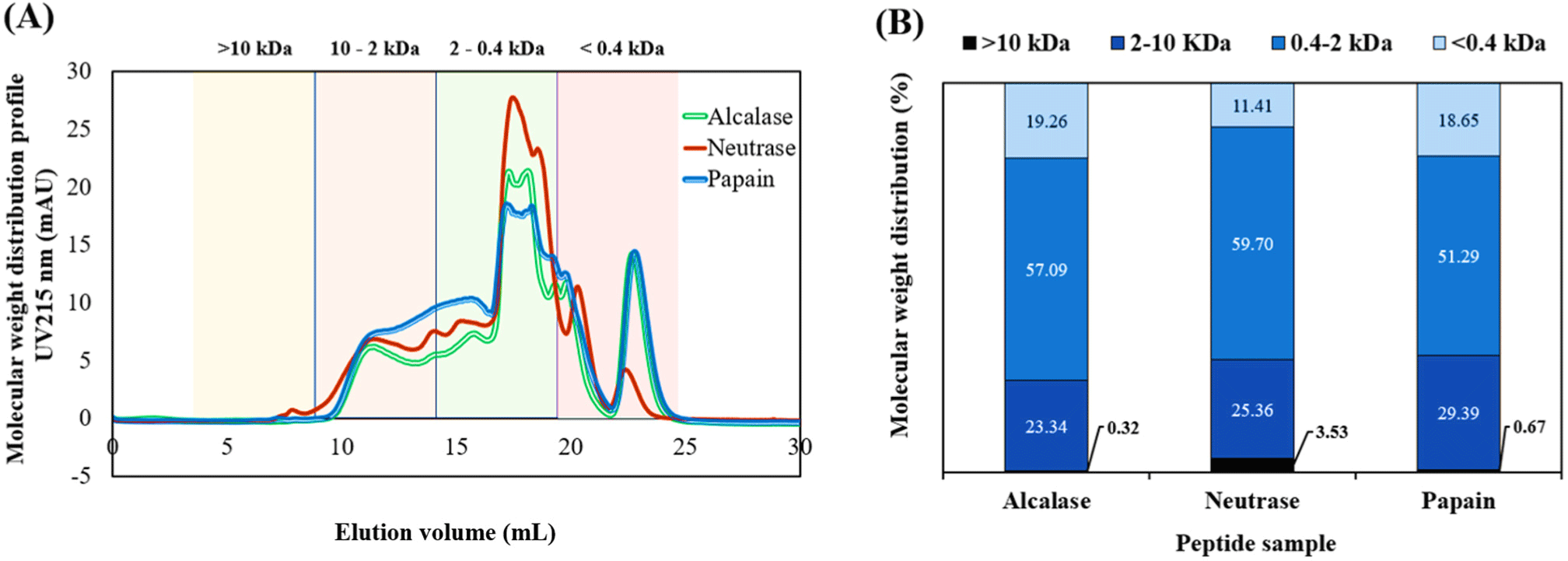
In evaluating the optimal hydrolysis conditions, we considered three key criteria: bioactivity, protein recovery, and salt interference. The peptide derived from alcalase hydrolysis demonstrated the highest level of protein recovery. On the other hand, the peptide resulting from neutrase hydrolysis exhibited the strongest antioxidant activity. Even though the protein recovery in neutrase hydrolysis was 8% lower than that in alcalase hydrolysis, we gave precedence to bioactivity in our selection process. It’s also worth noting that the peptide from alcalase hydrolysis contained sodium chloride salt, which was used for pH adjustment during the process. Considering all these factors, the peptide derived from neutrase hydrolysis emerged as the most functional and suitable choice for the production of BB peptides, despite its slightly lower protein recovery rate. This decision was primarily driven by its superior bioactivity and the absence of salt interference.
UF is a membrane-based separation technique that fractionates solution components based on their molecular size. In this study, we utilized UF to partially concentrate the BB-N, a peptide that demonstrated the highest antioxidant activity, as shown in Fig. 4. We employed UF membranes with molecular weight cutoffs of 10 kDa and 3 kDa, resulting in three distinct fractions: >10 kDa, 3–10 kDa, and <3 kDa. Sequential UF revealed that the fraction with peptides smaller than 3 kDa had the highest protein recovery, as depicted in Fig. 6. This finding aligns with a previous study on sericin peptides derived from neutrase hydrolysate (Sangsawad et al., 2022).
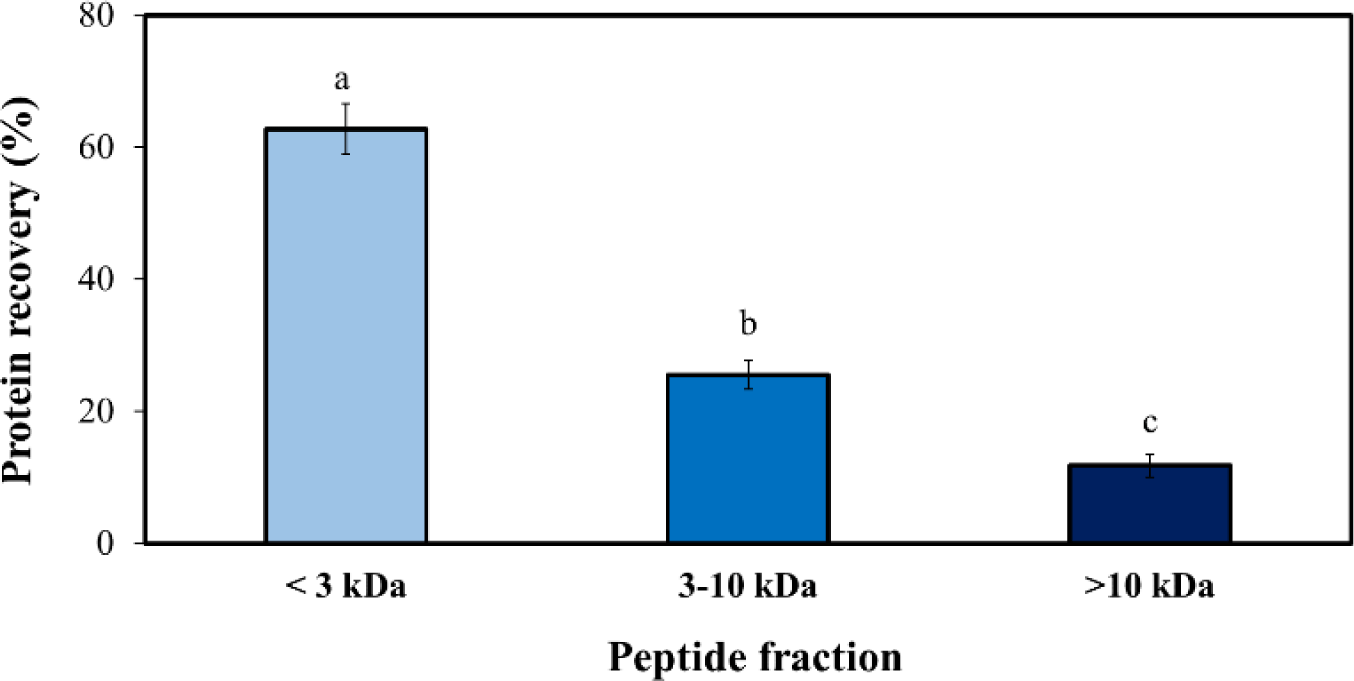
The permeate of the UF-3 kDa fraction exhibited the highest antioxidant activities, as determined by ABTS radical scavenging, FRAP, and metal-chelating assays (p<0.05, Figs. 7A and C). This result supports our hypothesis that peptides smaller than 3 kDa possess superior antioxidant capabilities. It underscores the potential of the <3 kDa fraction as a rich source of bioactive peptides. Moreover, this fraction contained the largest quantity of peptides, suggesting its suitability for use in a process to concentrate the BB-N peptide, which is known for its remarkable antioxidant properties.
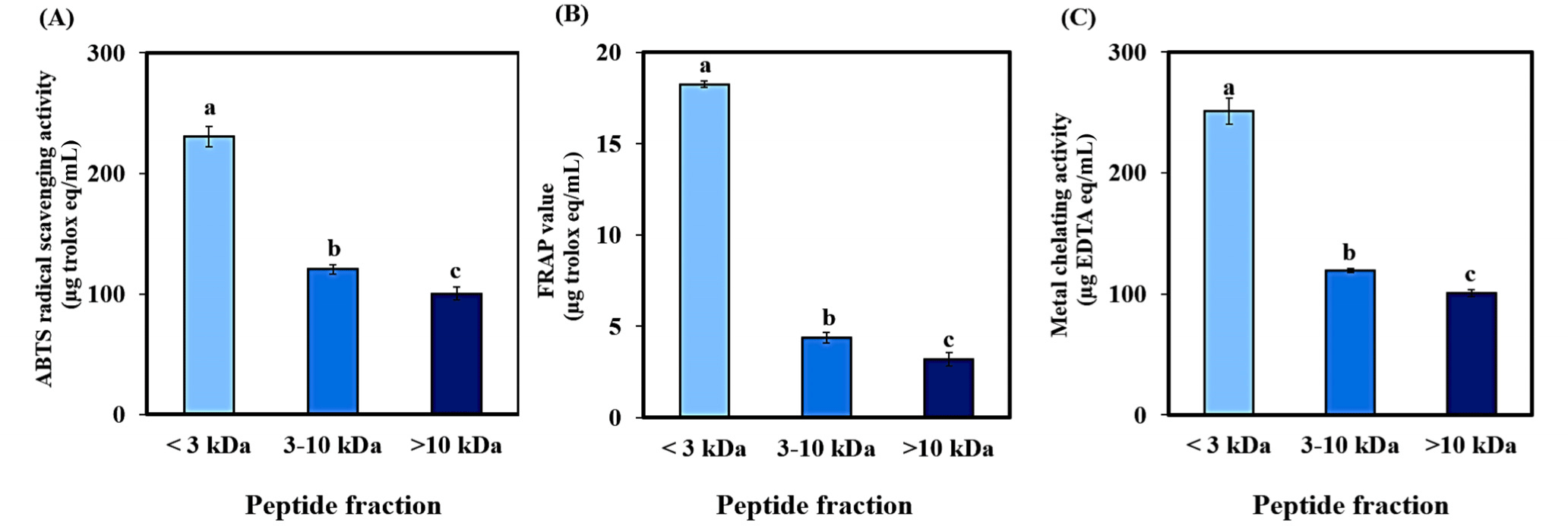
Our findings are consistent with a study on the kawakawa fish peptide (Taheri and Bakhshizadeh, 2020), which generated potent antioxidant activities in peptide fractions below 3 kDa. However, our study is unique as it is the first to report the antioxidant activity of bovine blood peptides derived from neutrase hydrolysis and to demonstrate the impact of UF on its partial concentration. Consequently, our research offers innovative insights into the production and concentration of bovine blood peptide, highlighting its potential as a valuable functional food ingredient with potential health benefits.
In this study, SDS-PAGE, a technique widely employed for separating proteins based on their molecular weight, was used to examine the protein profiles of bovine blood in its raw and modified (cooked and digested) states. Fig. 8 shows the SDS-PAGE results for various BB samples. Three distinct bands appear in Lane 1, which corresponds to raw BB. These bands represent protein complexes of fibrinogen, albumin, globulin, and hemoglobin monomer. In contrast, Lane 2, which represents cooked BB, shows denatured proteins with reduced intensity in the albumin, globulin, and fibrinogen bands. However, the intensity of the hemoglobin monomer band remains unchanged.
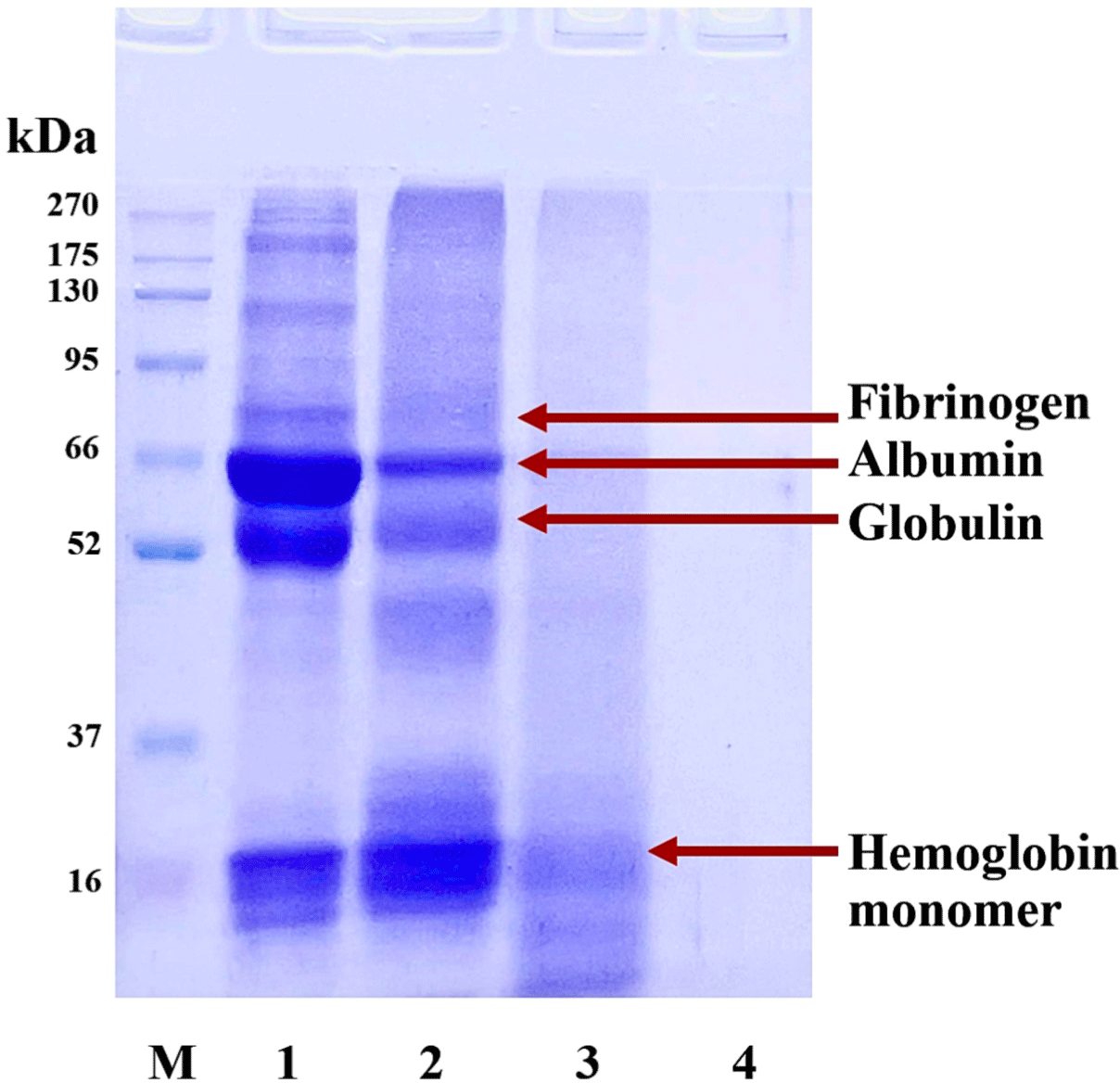
Our study collected bovine blood without adding sodium chloride or sodium citrate, an anticoagulant. This process allowed the blood to retain its native form of fibrinogen, a key protein in blood coagulation. Due to its complex structure, fibrinogen resists protease hydrolysis (Tanka-Salamon et al., 2010). During the production of bovine blood (BB), a vital step involved subjecting the blood to thermal treatment at 90°C for 30 min. This process is aimed to enhance the potential hydrolysis of BB by promoting the degradation of fibrinogen. Our analysis revealed fibrinogen degradation in Lane 2. To delve deeper into the fibrinogen degradation process, we examined the precipitate or insoluble fraction of BB-N hydrolysis in Lane 3. The absence of a fibrinogen band in this lane strongly suggests that fibrinogen had indeed been degraded and hydrolyzed. These findings shed light on the structural preparation of BB proteins before undergoing hydrolysis, offering valuable insights for further research and development.
Finally, Lane 4 displays the profile of the BB-N peptide (crude peptide). This sample does not show any visible protein bands, indicating that all proteins and peptides in this sample have an MW lower than 16 kDa. This finding supports the idea that the cooking process, which potentially causes fibrinogen, albumin, and globulin degradation, facilitates the hydrolysis of BB proteins.
This experiment is aimed to identify the peptide sequence derived from the permeate of UF-3 kDa of BB-N. This process was achieved through a systematic process of isolation and identification. SEC was used to divide the peptide into three fractions (F1, F2, and F3), each of which revealed distinct antioxidant activity, as shown in Figs. 9A and B. Fraction F2 was notable, as it displayed the highest peptide yield at 65% and the greatest antioxidant activity among the three fractions. Subsequent analysis revealed that F2 predominantly contained 900 Da and 720 Da peptides, as indicated by broad and intense peak patterns. Remarkable antioxidant activities were demonstrated by this specific fraction, surpassing the original permeate of UF-3 kDa in ABTS radical scavenging, FRAP assays, and metal chelating activity. This result confirmed an enhanced activity compared to the original fraction.
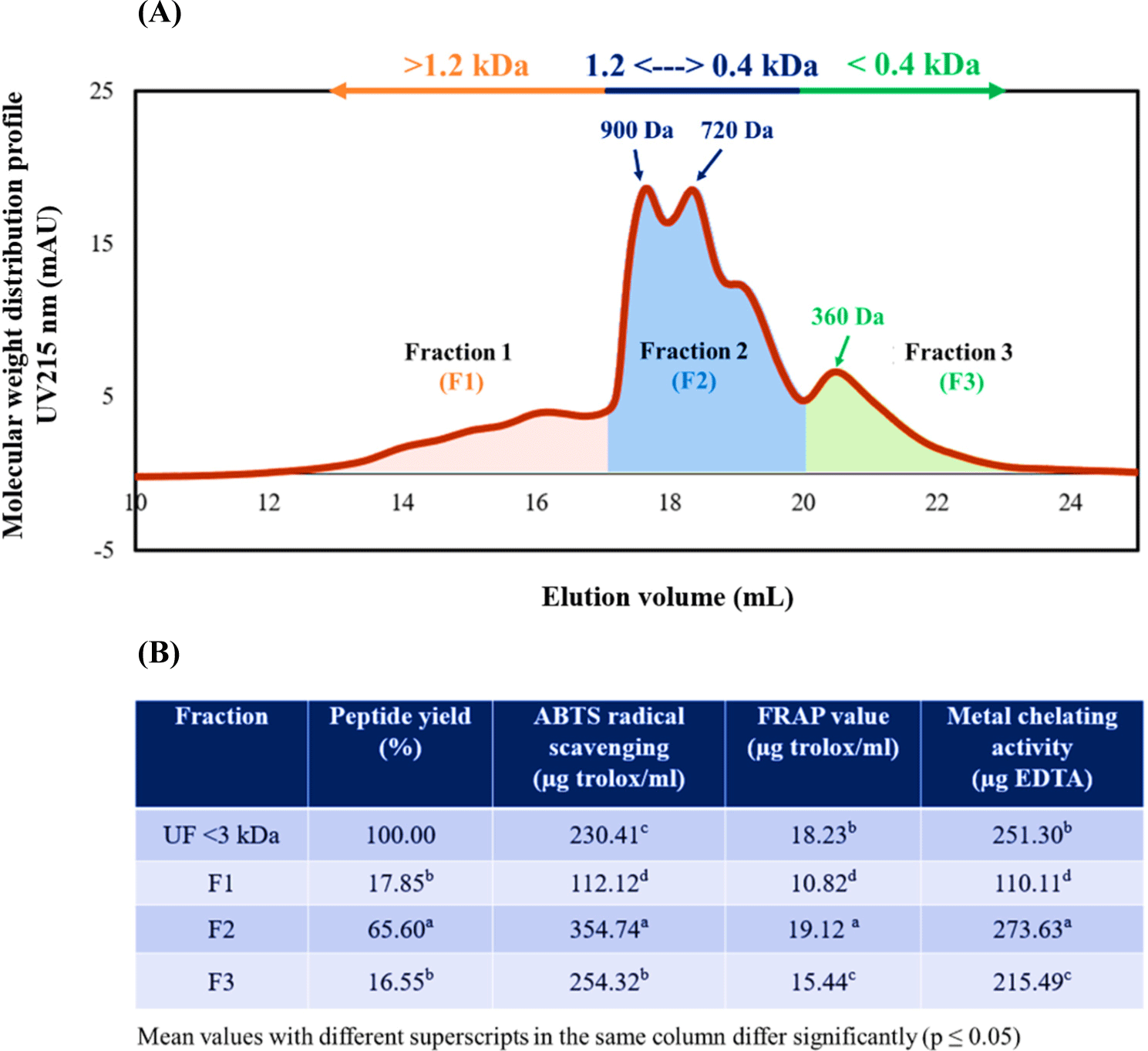
Although antioxidant peptides were also contained in F1 and F3, their relatively lower activity led to their exclusion. Consequently, the focus was placed on fraction F2 to identify the antioxidant peptide sequence. Unraveling this peptide sequence could provide insights into the structure-activity relationship of antioxidant peptides derived from BB proteins, a relationship that is not yet fully understood.
Table 2 displays the sequences of nine newly discovered peptides extracted from fraction F2. These peptides hold importance as they unveil previously undocumented sequences of antioxidant peptides. These sequences were discovered during an exhaustive exploration of a bioactive peptide database derived from food, accessible at http://www.cqudfbp.net/index.jsp. The peptides vary in length, with sequences ranging from four to six amino acids, indicating their diversity. The identification process utilized de novo sequencing, resulting in high scores that surpassed 70%, thereby confirming the accuracy of the identification. Interestingly, these peptides resemble hemoglobin proteins found in bovine blood, as the UniProt protein databases indicated. To illustrate, Fig. 10 showcases the MS/MS spectra of one such peptide, IAWGK. This example serves to further our understanding of these newly discovered peptides.
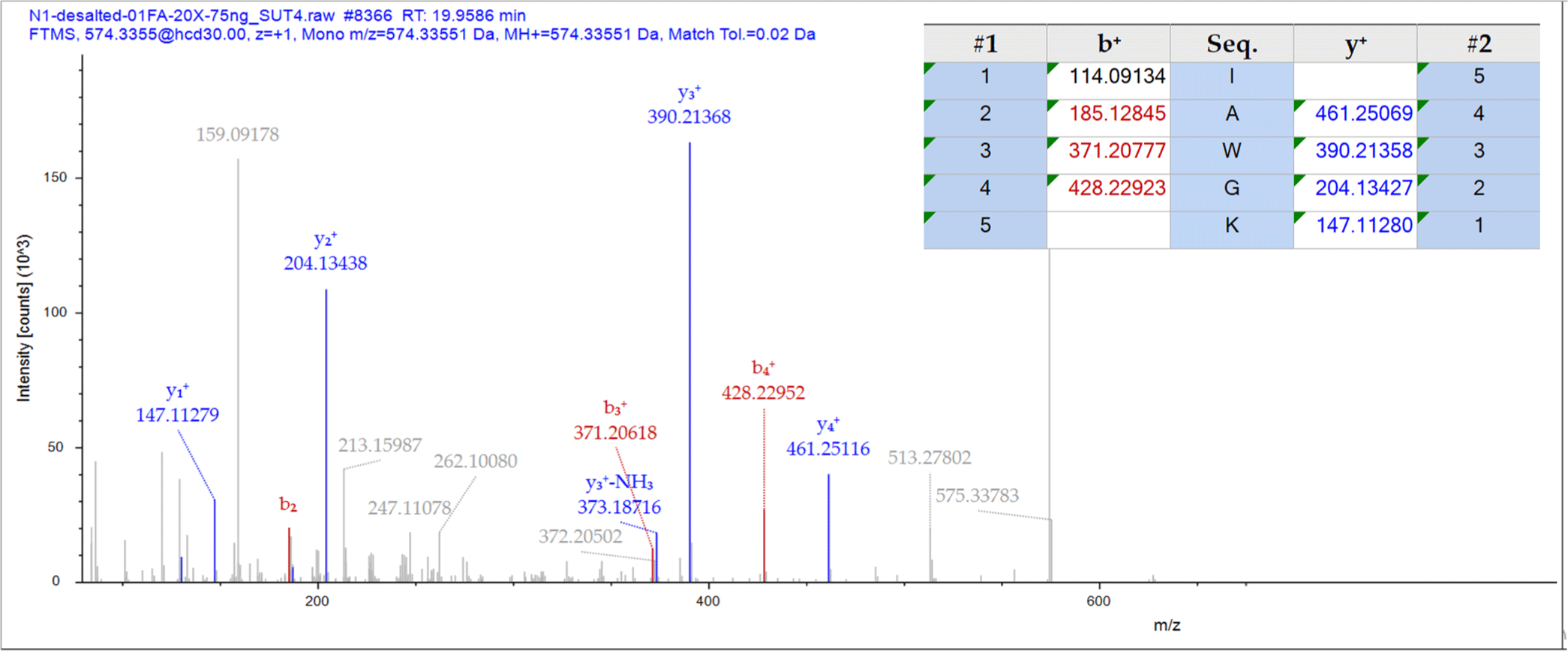
The nine peptides under study are composed of various combinations of 16 distinct amino acids: Ala, Asp, Glu, Phe, Gly, Ile, Lys, Leu, Met, Asn, Pro, Gln, Arg, Thr, Val, and Trp. Three different assays were employed to evaluate these peptides’ antioxidant activity: ABTS, FRAP, and metal chelating. The effectiveness of the peptides was measured using the IC50 value, which represents the peptide concentration required to inhibit 50% of the oxidation reaction. A lower IC50 value signifies stronger antioxidant activity. Table 2 provides a detailed overview of the antioxidant activity of the peptides, which varies depending on the assay method used. For instance, the peptide IAWGK exhibits the most potent ABTS radical scavenging activity, with an IC50 value of 0.28 mg/mL. It is followed by the peptides VDLL and MTTPNK. In the FRAP assay, IAWGK and VEDVK display the strongest activity, with IC50 values of 0.63 and 0.40 mg/mL, respectively. They are followed by the peptides MPLVR and VDLL. Regarding metal chelating activity, IAWGK again demonstrates the strongest activity with an
IC50 value of 2.40 mg/mL. It is followed by the peptides MTTPNK, MPLVR, TVIL, and KIII. Considering the results from all three assays, IAWGK emerges as the peptide with the most potent antioxidant activity. Furthermore, these findings suggest that different peptides may operate through other mechanisms of action or have varying affinities for various oxidants.
To comprehend the structure and activity of these peptides, we can refer to previous reports on the structure-activity relationship of antioxidant peptides derived from various proteins, as documented by Nwachukwu and Aluko (2019) and Zou et al. (2016). Take the peptide IAWGK, for example. It contains aromatic (W) and hydrophobic (I and A) amino acids known to enhance antioxidant activity. Due to their indole ring structure, the peptide includes tryptophan residues, effective radical scavengers. A positively charged lysine residue at the C-terminus may also boost its metal-chelating ability by forming complexes with metal ions. Therefore, the presence of I, A, W, and K (at the C-terminal) in IAWGK aligns with the sequence characteristics of antioxidant peptides reported in previous studies. On the other hand, the peptide VEDVK, despite containing hydrophobic amino acids (V), exhibits the highest IC50 value for the ABTS assay, indicating the lowest activity for this test. However, it demonstrates the strongest antioxidant activity for the FRAP assay, as indicated by its lowest IC50 value. This discrepancy could be attributed to the peptide sequence’s specific arrangement of amino acids, underscoring the importance of the structure-activity relationship in determining antioxidant activity.
As illustrated in Table 2, the length and composition of peptides significantly impact their antioxidant activity. Generally, shorter peptides, consisting of 4–5 amino acids, exhibit lower IC50 values, indicating a higher antioxidant activity than longer peptides composed of 6 amino acids. However, there are exceptions to this trend. For instance, peptides IAWGK and VEDVK, despite having the same length, display different IC50 values. This observation suggests that the type and order of amino acids play a crucial role in determining antioxidant activity. The IC50 values obtained in this study are comparable to those reported for other antioxidant peptides derived from various sources. For example, the peptides LSPGEL, VYFDR, and PGPTY from gracilariopsis lemaneiformis have IC50 values ranging from 0.24 to 5.35 mg/mL for ABTS activity (Hu et al., 2022). Similarly, rice peptides PNYTDA, TSQLLSDQ, TRTGDPFF, and NFHPQ exhibit IC50 values between 0.08 and 0.24 mg/mL for FRAP activity (Yan et al., 2015). Furthermore, palm kernel cake peptides YLLLK, GGIF, WAFS, AWFS, LPWRPATNVF, and GVQEGAGHYALL show IC50 values from 0.2 to 2.3 mg/mL for metal chelating activity (Zarei et al., 2014). Our study’s comparisons indicate a promising antioxidant potential, particularly for the peptide IAWGK among the peptides examined. In summary, this research is groundbreaking as it marks the initial identification and exploration of the potent antioxidant properties of IAWGK and eight other peptides derived from bovine blood. The insights gained from the structure-activity relationship of these peptides provide valuable guidance for developing even more potent antioxidant peptides.
Bioactive peptides, known to undergo hydrolysis during GI digestion, have garnered significant interest in nutrition science (Xu et al., 2019). As depicted in Table 3, nine specific peptides were subjected to simulated in silico GI digestion, resulting in various fragments. Six peptides - IAWGK, VDLL, MPLVR, LPQL, TVIL, and DFPGLQ – were hydrolyzable into dipeptides and tripeptides. These hydrolyzed fragments have been reported to possess several health-promoting properties, including antioxidant activity, ACE inhibition (contributing to antihypertensive effects), and DPP-IV inhibition (contributing to type-II antidiabetic effects). On the other hand, the peptides MTTPNK, KIII, and VEDVK demonstrated resistance to hydrolysis, suggesting potential stability within the GI environment.
It is known that small intestinal epithelial cells can absorb peptides of up to six amino acid residues and subsequently transfer them into the bloodstream (Bouglé and Bouhallab, 2017). Furthermore, the bioactive peptides KPLLCS, ELFTT, and KPLL were subjected to absorption tests using the Caco-2 cell monolayer model. It has been reported that both the parent peptides and their fragments could be absorbed through the Caco-2 cell monolayer (Sangsawad et al., 2018a). Therefore, it is hypothesized that after consumption, the parent peptides, their fragments, and the intact forms of the nine peptides might retain their antioxidant activity at the target organ. The overall stages of deriving the antioxidant peptides and their predicted structural changes are shown in Fig. 11.
Regarding antioxidant properties of peptides and their potential in vivo effects, peptides known for their antioxidant properties, specifically rapeseed protein hydrolysate LY, RALP, and GHS, have been observed to enhance the activity of superoxide dismutase and reduce malondialdehyde levels in rat plasma when administered at a dose of 30 mg/kg (He et al., 2019). These peptides showcase their antioxidant capabilities by effectively preventing the generation of free radicals and the oxidation of unsaturated fatty acids in vivo. In addition, the tripeptide LLY, identified in tryptic digests of milk (β-casein), has exhibited antioxidant properties by scavenging ABTS radicals. This result was observed both in vitro and in vivo within Swiss Albino mice exposed to ethanol-induced oxidative stress (Sowmya et al., 2018). Notably, this tripeptide increased the concentration of reduced glutathione in the liver and upregulated the expression of liver genes associated with ethanol-induced stress. Simultaneously, it decreased the levels of malondialdehyde and reduced the activities of antioxidant enzymes. In our research, we discovered nine new peptides, particularly IAWGK, which were characterized by short sequences and demonstrated potent in vitro antioxidant activity. The peptides identified in our research may exhibit strong antioxidant properties when tested within a living organism or in vivo. However, additional studies conducted in vivo are necessary to confirm these properties and further investigate the extent of their effects. It will provide a more comprehensive understanding of their antioxidant capabilities and potential health benefits.
Conclusion
This research represents a groundbreaking initiative to investigate antioxidant peptides derived from unaltered bovine blood without using salt as an anticoagulant. The optimal conditions for peptide generation were established as 32.5% protein, 5% neutrase (E/S), and a hydrolysis period of 4 h. Upon concentration using UF-3 kDa, the resulting permeate displayed the highest concentrations of antioxidants and proteins. Through a series of identification procedures, we discovered nine novel antioxidant peptides: IWAGK, VDLL, MTTPNK, MPLVR, KIII, LPQL, TVIL, DFPGLQ, and VEDVK. Among these, IWAGK demonstrated the most potent antioxidant activities. We further conducted simulated in silico GI digestion. As a result, IWAGK, VDLL, MPLVR, LPQL, TVIL, and DFPGLQ showed the potential to break down into bioactive dipeptides and tripeptides. In contrast, MTTPNK, KIII, and VEDVK exhibited resistance, suggesting a potential to circulate through the bloodstream and function at the target organs. Our study highlights the promising potential of BB-N as a novel dietary ingredient, showcasing its antioxidant properties linked to various health benefits. However, further research, including animal and human feeding experiments, is crucial to fully understand the therapeutic effectiveness of BB peptides derived from neutrase hydrolysis.













