Introduction
Ice cream is a complex food colloid containing emulsion, foam, and dispersed ice crystals in a viscous aqueous solution. Ice cream generally contains about 0.1%–0.3% emulsifiers, which contribute to the aeration of ice cream (Goff, 1997a). Monoglycerides, monoglyceride derivatives, and polysorbates have been commonly used as ice cream emulsifiers, and these emulsifiers are selectively adsorbed to the oil-water interface over milk proteins due to their greater surface activity (Euston and Goff, 2019). This preferential adsorption of emulsifiers leads to protein displacement at the interface and induces partial destabilization of fat droplets during aging (Pelan et al., 1997). The fat globule network formed by partial coalescence stabilizes air cells (Goff, 1997b) and improves overrun, melt resistance, and shape retention of ice cream (Barford et al., 1991; Daw and Hartel, 2015). Thus, ice cream mixes without suitable emulsifiers tend to have a wet and weak body due to insufficient partial coalescence (Goff, 1997a). In this regard, the adsorption behaviors of emulsifiers onto fat surfaces followed by protein displacement, provide valuable insights for the prediction of ice cream quality.
Recently, there has been a growing demand to replace synthetic food additives with more natural and healthy ones. Not all but some emulsifiers, such as carboxymethylcellulose and polysorbate 80, influence the interactions of mucus with gut microbiota and increase the risk of gut inflammation in mice and ex vivo human microbiota models (Chassaing et al., 2015; Naimi et al., 2021).
Milk polar lipids (MPL) are constituents of milk fat globule membrane (MFGM) and encompass glycosphingolipids, glycerophospholipids, sphingolipids, and gangliosides (Bourlieu and Michalski, 2015). Prophylatic MFGM administration improved mucus barrier function and attenuated acute colitis in a mice model of inflammatory bowel disease by decreasing inflammatory cytokines (Wu et al., 2022). The administration of polar lipid-enriched MFGM also improved obesity-mediated glucose metabolism disorders in rats (Li et al., 2022). These results suggest that MPL intake has beneficial effects on the modulation of gut microbiota.
The interactions of MPL and dairy proteins are complex, and the effect on emulsion stability can vary depending on the type and concentration of proteins (Ahn et al., 2022). Milk phospholipid concentrate was able to competitively displace proteins from the surface of whey protein isolate (WPI)-stabilized emulsion (Livney et al., 2017). These studies suggest that interactions between MPL and milk proteins lead to partial coagulation of fat droplets by limiting protein layer formation at the emulsion interface. However, various single-ingredient emulsifier substitutes such as phospholipid-enriched whey protein concentrate, citrus fibers, rice protein contrate, and lupine protein concentrate did not achieve acceptable ice cream quality compared to mono- and di-glycerides (MDG), which are commonly used as ice cream emulsifier (Loffredi et al., 2021). The authors suggest that a combination of different ingredients may be a promising strategy to replace MDG.
Protein hydrolysates generally display a better affinity for the oil/water interface than corresponding intact proteins because of increased hydrophobic amino acids exposure and molecular flexibility. We hypothesize that an appropriate combination of MPL and casein hydrolysate (CH) will improve partial coalescence and overrun in ice cream as well as provide a health benefit due to MPL. To prove this hypothesis, the effects of a combined MPL and CH (MPL+CH) formulation on protein displacement, emulsion stability, and interfacial tension were analyzed using a model milk protein-stabilized emulsion. Finally, the effects of this emulsifier combination on ice cream characteristics, such as microstructure, overrun, and melting, were evaluated.
Materials and Methods
Diacetyl tartaric acid esters of monoglycerides (DATEM; Danisco, Copenhagen, Denmark), MPL (DS-WPL 25; >25% phospholipid; Solus Biotech, Seoul, Korea), and CH (90% protein: 9% degree of hydrolysis, molecular weight distribution: 55.9%<5,000 Da; 37.5% 5,000–20,000 Da; 6.6%>20,000 Da; Tatua, Morrinsville, New Zealand) were used as emulsifiers. Sodium caseinate (85% protein) and WPI (89% protein) were obtained from Lactoprot (Kaltenkirchen, Germany) and Lactalis Ingredients (Bourgbarré, France), respectively. Medium chain triacylglycerol (MCT) was purchased from Danisco. Ingredients used for ice cream preparation, including milk cream (Seoul Dairy, Seoul, Korea), skim milk powder (Seoul Dairy), corn syrup (Daesang, Seoul, Korea), and sugar (Samyang, Seoul, Korea) were purchased from a local super market in Seoul, Korea.
Milk protein (1%, w/v, sodium caseinate: WPI=8:2) or milk protein and CH (0.01%/0.05%/0.1%, w/v) were dispersed in sodium phosphate buffer (10 mM, pH 7.0) while MPL (1%, w/v) and DATEM (1%, w/v) were dispersed in MCT oil. After mixing the oil and protein phase, the mixture was blended using an Ultra-Turrax T25 homogenizer (IKA-Werke, Staufen, Germany) at 8,000 and 13,000 rpm for 1 min, respectively. The coarse emulsion was subjected to high-pressure homogenization using an NLM 100 Nano Disperser (Ilshin Autoclave, Daejeon, Korea) at 18/4 Pa. The emulsions were immediately cooled in an ice bucket and then stored at 4°C.
Ice cream mixes containing different emulsifiers were prepared, as shown in Table 1. After blending, the ice cream mix was pasteurized (72°C, 3 min) and passed through a 2-stage homogenizer (GEA Niro Soavi, Parma, Italy) at 18 MPa for the first stage and 3.4 MPa for the second stage. The homogenized mix was cooled in ice water and aged in a refrigerator at 4°C for 18 h. Ice cream was made using an ice cream machine (ISI-151TGN, Icetro, Incheon, Korea). Ice cream was hardened in a deep freezer (–80°C) for 6 h and stored in a freezer at below –20°C.
The volume-weighted mean diameter [D(4,3)] of the emulsion was measured 1 h after emulsion formation using a Horiba LA-960 (Horiba Instruments, Kyoto, Japan). Refractive indices of oil (1.520) and deionized water (1.330) were used for calculation. The particle size distribution of aged ice cream was analyzed after appropriate dilution with distilled water. In the ice cream, the cumulative percentage of particles >4.0 μm was considered as the index of partial fat coalescence because no globules >4.0 μm were present in the mix (Bolliger et al., 2000). All determinations were carried out in triplicate.
The centrifugal stability constant was determined using the method of Liu et al. (2022) with some modifications. After 1 h of aging at 4°C, emulsions (2 mL) were centrifuged at 3,000×g for 15 min. The supernatant (0.5 mL) was obtained after dilution (20-fold with distilled water) followed by centrifugation. The absorbance (A) was measured at 450 nm using a UV–visible spectrophotometer (Ultrospec 2100 pro, Amersham Biosciences, Uppsala, Sweden). The absorbance of the emulsion without centrifugation (A0) was recorded, and the centrifugal stability constant (KE) was calculated using the following formula:
The interfacial tension between the protein solution and oil was determined according to the pendant drop method using KRÜSS drop-shape analyzer (DSA 25; KRÜSS, Hamburg, Germany). Emulsifiers (milk protein, DATEM, MPL, CH, and MPL+CH) were solubilized in the aqueous phase, and MCT oil was used as the oil phase. The concentrations of protein and emulsifiers were the same as emulsion as described in the emulsion preparation. A syringe needle with a diameter of 1.825 mm was used to create a pendant drop of MCT oil. Individual oil drop images were obtained using a high-speed digital camera, and interfacial tension (mN/m) was calculated from the shape of the drop using the Young−Laplace equation. Interfacial tension was determined at ambient temperature in triplicate.
The protein displacement of the model emulsion was determined using the method of Chen et al. (2019a) with a slight modification. Samples (30 mL) were centrifuged (15,000×g, 45 min, 4°C) and the cream layers were recovered after carefully separating the aqueous phase. In the case of ice cream, cream layers were separated after thawing ice cream (4 mL) and an additional washing step was conducted with distilled water. The adsorbed protein content in the cream layers (fat globule surface protein) was calculated as the difference between the initial and aqueous protein content. The protein content was quantified by the bicinchoninic acid assay (Smith et al., 1985). Protein displacement was expressed using the following formula:
M0: amount of fat globule surface protein in the absence of emulsifier (mg), M1: amount of fat globule surface protein in the presence of emulsifier (mg).
The separated cream layers were dispersed in sodium phosphate buffer (100 mM, pH 7.0) and heated at 95°C for 20 min. The samples were mixed with the sample buffer and then boiled for 3 min and centrifuged (12,000×g, 5 min) to remove residual fat. The samples (20 μL) were loaded onto a 12% acrylamide gel (TGX Stain-FreeTM FastCastTM Acrylamide Kit, Bio-Rad Laboratories, Hercules, CA, USA), and electrophoresis was conducted at a voltage of 80 V. The gel was visualized using a ChemiDocTM XRS+System and relative intensity of αs- and β-casein was quantified by Image LabTM Software (ver. 5.1; Bio-Rad Laboratories).
Ice cream mix and ice cream were filled in the same container (280 mL) and overrun was measured based on the weight difference between ice cream mix and frozen ice cream using the following formula:
The melting rate of hardened ice cream was determined using the screen drip-through test (Muse and Hartel, 2004). The samples (72 g) were placed on a 20-mesh grid and allowed to stand at ambient temperature (25°C). The liquified samples that dripped through the grid were collected and their weight was recorded every 5 min for 70 min. The melting rate was calculated by plotting the drip weight as a function of time, and the slope of the linear part of the curve was expressed. The melting rate test was determined in triplicate.
The microstructure of thawed ice cream was observed using confocal laser scanning microscopy (CLSM; Leica, Heidelberg, Germany), as described by Lee et al. (2023). Fat and protein were stained with Nile Red and Fast Green (0.1% and 0.01% in ethanol), respectively. For image analysis, samples (200 μL) were immobilized by the addition of agarose solution (0.5%, 1:1, v/v) and visualized as previously described (Ahn et al., 2022).
All analytical assays were carried out at least in triplicate. Data are presented as mean±SD. Statistical differences were analyzed by one-way analysis of variance (ANOVA) using SPSS software (ver. 26 for Windows, IBM, Armonk, NY, USA). If ANOVA indicated a significant difference (p<0.05), Tukey’s multiple comparison test was used to compare significant differences between treatment means.
Results and Discussion
The mean particle diameters of milk protein (casein:whey=8:2)-stabilized emulsions (control emulsion) with or without emulsifiers are shown in Table 2. The mean particle diameter of the control emulsion was significantly decreased upon the addition of DATEM or MPL, respectively (p<0.05). There was no significant difference in the mean particle diameter between the DATEM and MPL emulsions. The effect of CH addition on the mean particle diameter of emulsions varied depending on the additive level. CH at a low concentration (CH_L, 0.01%, w/v) did not affect the mean particle diameter, whereas CH at a high concentration (CH_H, 0.05%, w/v) significantly increased the mean particle diameter from 1.67 to 2.80 μm. The combination of MPL and CH decreased the mean particle diameter of emulsions compared to that of the CH counterpart, but the addition of MPL+CH_H increased the mean particle diameter compared to that of the control emulsion.
Centrifugal stability constants are calculated by the difference in absorbance before and after centrifugation of emulsions. Thus, a low stability constant denotes high emulsion stability. The emulsions containing CH_H with the larger particle size displayed a greater stability constant than the control emulsion. This result supports that the addition of high concentrations of CH caused significant destabilization of the emulsion.
The addition of DATEM (0.125%–0.250%) significantly decreased the mean particle diameter of whey protein-maltodextrin-stabilized emulsion and improved emulsion stability (Yu et al., 2021). Low molecular weight emulsifiers (LMWE) are able to locate at the interface together with protein, such as caseinate, and fill up the holes of the interfacial protein film until LMWE-mediated protein displacement take place (Munk et al., 2014).
Generally, limited enzymatic hydrolysis is applied in commercial protein hydrolysate production to minimize the risk of bitter taste development. Protein hydrolysis enhances surface adsorption kinetics by increasing exposure of hydrophobic groups (Chen et al., 2019b). Resistance of fat droplets to coalescence tends to be positively correlated with the amount of peptide >2 kDa (van der Ven et al., 2001). Similarly, protein hydrolysates with a relatively shorter chain length (<5 kDa) form a weak interfacial film, resulting in low emulsion stability (Schröder et al., 2017). This is partially explained by the fact that extensive hydrolysis increases the aqueous solubility of peptides rather than their adsorption at the oil-water interface (Conde and Patino, 2007).
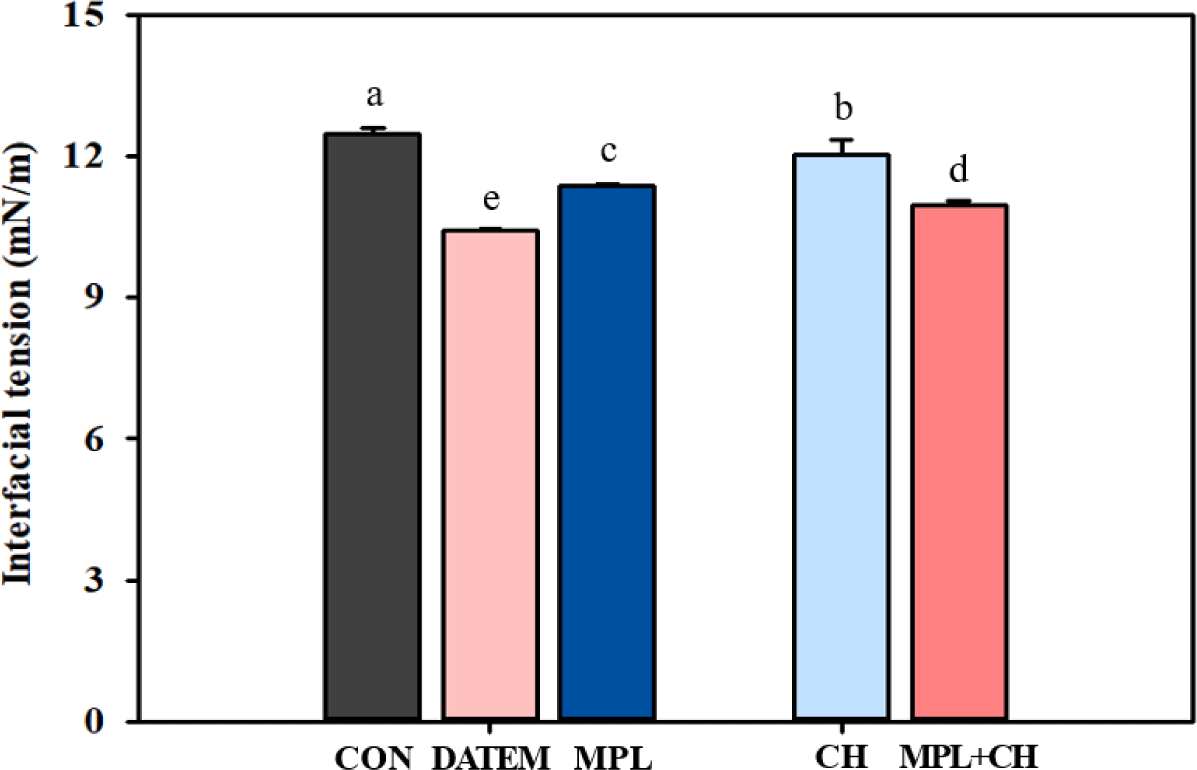
The changes in interfacial tension following the addition of DATEM, MPL, and MPL+CH were measured. Aqueous protein (control) decreased the interfacial tension by about 12 mN/m and the addition of DATEM (1%, w/v) and MPL (1%, w/v) significantly lowered the interfacial tension, suggesting DATEM and MPL can be preferentially adsorbed at the oil-water interface (p<0.05, Fig. 1). LMWE might fill up gaps in the interfacial protein network that are not covered by larger proteins (Mackie et al., 2000). DATEM actively reduced the interfacial tension of aqueous milk protein and displayed the greatest interfacial tension-lowering effect. The addition of CH also significantly reduced the interfacial tension even at a low additive level (0.01%. w/v). When MPL+CH was applied the interfacial tension was further decreased compared to MPL alone. Emulsifiers with high interfacial tension-lowering effect can be more densely packed at emulsion surface (Bezelgues et al., 2008). In this regard, MPL+CH facilitates the formation of a mixed interfacial MPL+CH layer, and the combined presence of MPL and CH leads to changes in interfacial viscoelasticity, affecting emulsion destabilization. Dalgleish et al. (1995) studied the surface interaction of casein and Tween 80 in an oil-in water emulsion. They found that the protein-emulsifier interactions not only affected the amount of adsorbed protein but also its conformation at the interface until equilibrium between aqueous protein and adsorbed protein was established.
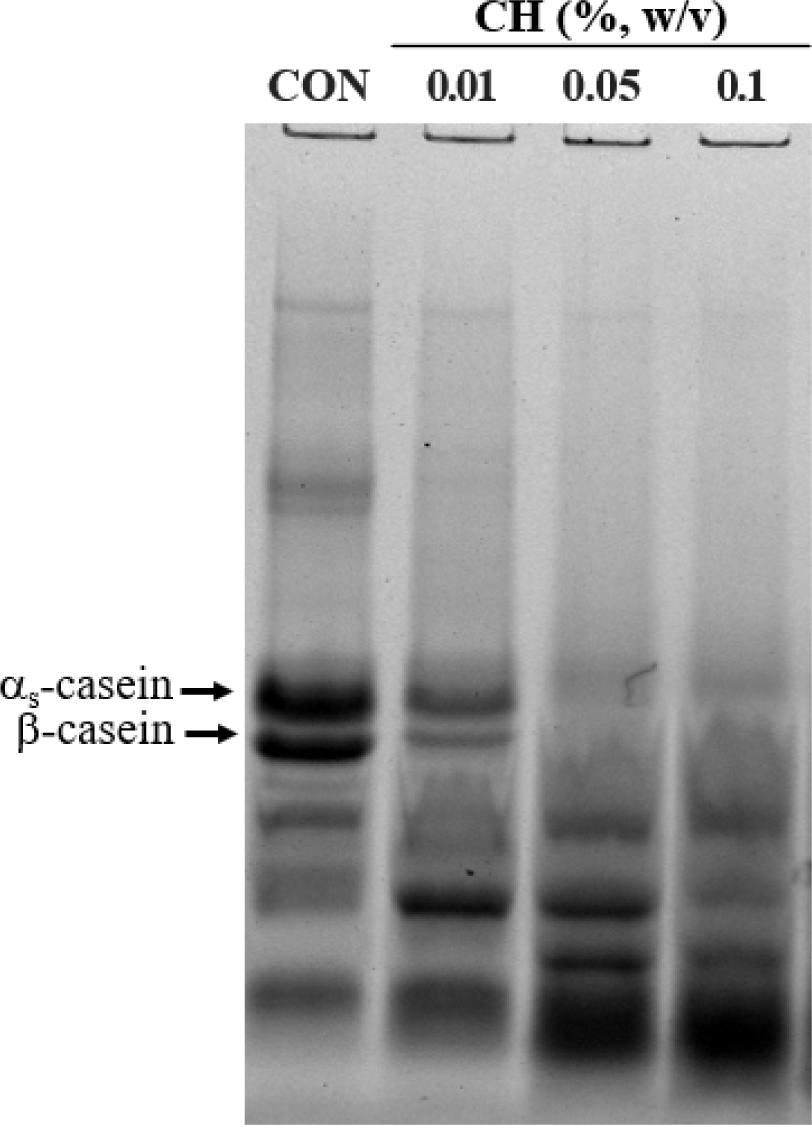
The addition of LMWE often leads to displacement of interfacial adsorbed proteins, followed by partial coalescence of fat globules (Goff, 1997b). The effect of emulsifier addition on milk protein displacement from cream layers was indirectly determined by quantification of aqueous proteins after cream separation, and profiles of protein remaining in the cream layers was examined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
The intensity of casein bands remaining in the emulsion cream layers decreased as the concentration of CH increased. Caseins in the cream layers was almost completely replaced by low-molecular-weight casein peptides when 0.05% or 0.1% CH was added. In addition, CH (0.01%) more effectively displaced β-casein than αs-casein (Fig. 2). β-casein is the most abundant and surface-active casein and adsorbed β-casein at the interface stabilizes an emulsion by providing thickness and steric hindrance (Li et al., 2016). The low emulsion stability observed with CH_H addition (Table 2) might be associated with the desorption of β-casein. Consequently, the combination of MPL+CH (>0.05%) was excluded from further ice cream application experiments because the emulsion in ice cream should have good stability in the stationary state before freezing and it provide sufficient instability under shear conditions during freezing in ice cream production (Goff, 1997b).
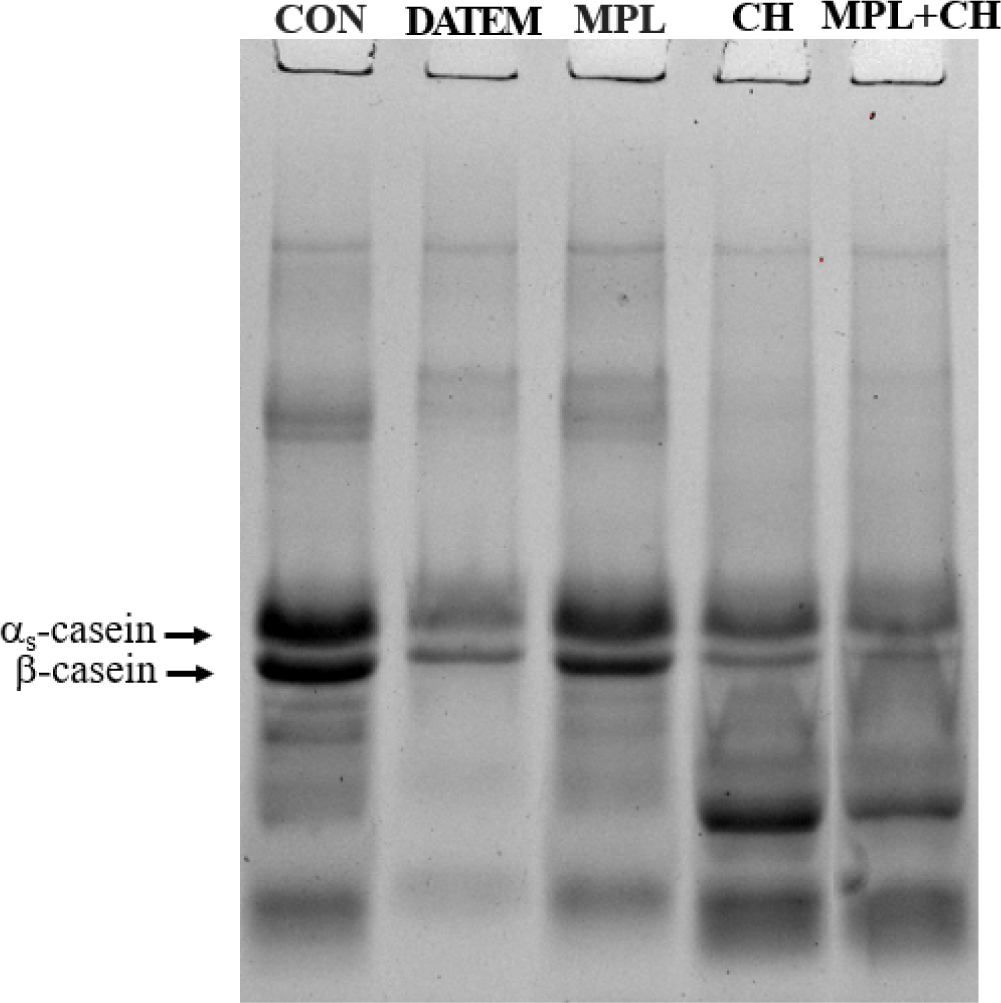
From the SDS-PAGE protein profile, the intensity of major casein constituents, αs and β-casein, was decreased by the addition of other emulsifiers (Fig. 3). DATEM more greatly expelled αs- and β-casein from the cream layer surface when compared with MPL. The intensity of SDS-PAGE bands corresponding to casein and CH was further decreased when the MPL+CH combination was used. The presence of both LMWE and proteins influences emulsion stability depending on the concentration and type of emulsifiers/proteins as well as the sequence of addition. LMWE diffuse without restriction above the melting temperature. Thus, an emulsion interface occupied by LMWE exert less surface mechanical properties than that of protein-rich interface (Wilde et al., 2004). DATEM has a better affinity for the oil surface and is more likely to displace caseins adsorbed at the oil-water interface. DATEM (melting point: ~45°C) crystalizes from melt to a stable α-crystal form at the interface at high surfactant concentration, and these crystals promote penetration of the interfacial film to enhance partial coalescence (Hong, 1998). In addition, repulsive forces between the net negative (–) charge of casein emulsion and that of carboxyl groups in DATEM at pH 7.0 facilitate casein dispersion from the interfacial surface. Electrostatic interaction between adsorbed milk proteins and phosphatidylcholine, one of the major constituents of MPL has been demonstrated (Allen et al., 2008).
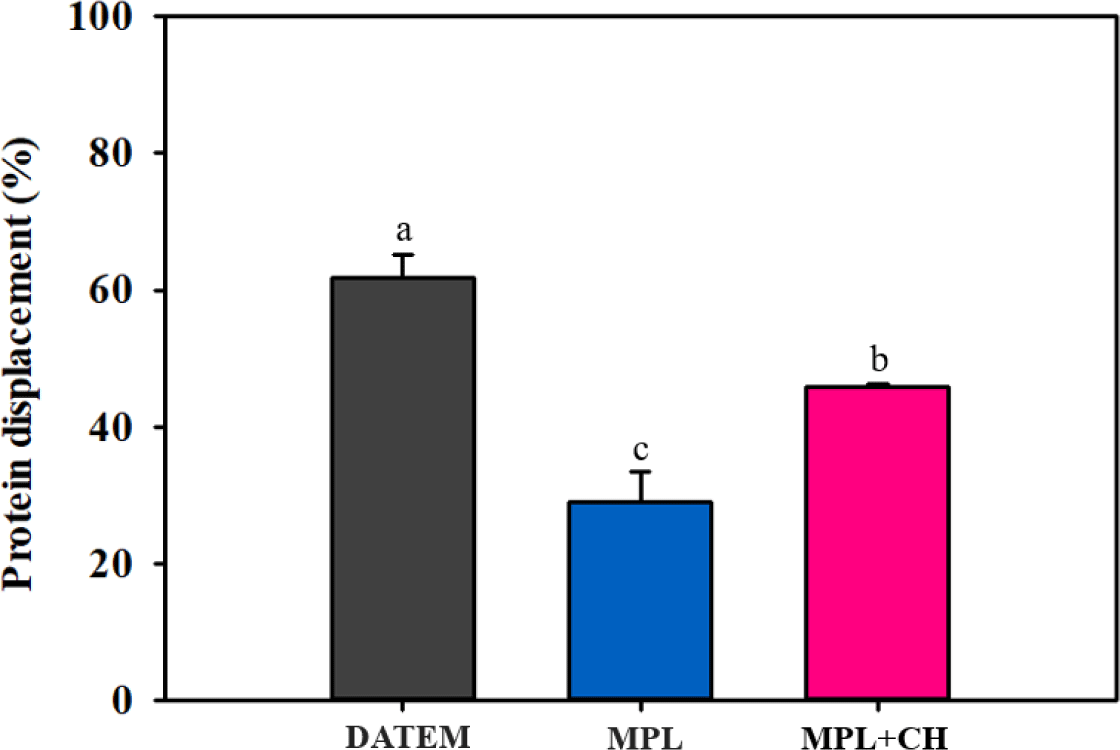
As shown in Fig. 4, the addition of DATEM (1%) more effectively displaced milk proteins from the cream layers when compared with MPL (62% vs. 29%). The combination of MPL+CH_L (0.01%) increased protein displacement (29% vs. 46%). Therefore, the combination of MPL+CH desorbed milk proteins at the interface in a synergistic manner without critically affecting stability in the static state.
During the homogenization of an ice cream mix, surface-active ingredients, such as proteins and emulsifiers, will adsorb at the oil-water interface. The formation of composite emulsion layers and the partial coalescence of fat play an important role in the quality attributes of ice cream. The concentration of MPL (0.15% vs. 0.3%) was selected to remain below the emulsifier concentration (about 0.5%) generally used in ice cream production, considering cost and sensory characteristics.
The addition of combined MPL+CH (L/M/H) significantly improved ice cream overrun (41%–43%) when compared with MPL alone (35%). The overrun of ice cream prepared with MPL (0.3%)+CH was comparable to that produced with DATEM (44%) as the emulsifier (Table 3). Higher overrun indicates that air is well distributed and retained in the ice cream structure. The increased overrun observed with the combination of MPL+CH is likely due to enhanced protein displacement from the emulsion surface, as shown in Fig. 4. CH, which has a relatively low MW and a flexible structure, may competitively adsorb at the oil-water interface, thereby reducing the surface coverage casein micelle. The decreased steric stabilization, resulting from limited casein micelle adsorption, leads to the formation of partially coalesced fat networks that confer excellent air cell stabilization property (Goff, 2016). In ice cream structure formation, LMWE gradually displaces milk proteins from the fat globule surfaces during freezing and develops fat globule clusters to stabilize foam structure (Warren and Hartel, 2018). Chen et al. (2019a) reported that protein displacement from the fat surface decreases the mechanical strength of the adsorbed layers and leads to partial coalescence fat globules. Under shear conditions, protein displacement is dependent on the type and concentration of emulsifiers (Davies et al., 2001).
LMWE not only displace proteins from the fat surface but also play an important role in fat crystallization. Water insoluble emulsifiers such as MDG, act as templates for fat crystallization, whereas water soluble Tween 80 forms loosely packed weak crystals (Rizzo et al., 2015). Fat crystals are able to penetrate interfacial layers and bridge fat droplets to form fat globule clusters and improve overrun. In this regard, MPL promotes fat crystal penetration with relatively weak protein displacement activity, while CH further facilitates protein desorption from the ice cream emulsion interface.
Differences in the melting rate of ice cream samples hardened for 6 h were compared. The melting rate of ice cream prepared with different emulsifier formulations did not show significant difference compared to control ice cream containing DATEM. It is known that ice cream with low overrun tends to melt quickly because air cells in ice cream act as a barrier against heat transfer (Muse and Hartel, 2004). The three-dimensional fat globules network stabilizes air cells by attachment to the bubble surfaces in ice cream and prevents serum drainage during melting, whereas ice cream with a less developed fat network exhibits rapid serum dripping (Koxholt et al., 2001; Wu et al., 2019).
The percentage of fat globules >4 μm was used as an index of the extent of partial coalescence. The ice cream prepared with MPL (0.3%)+CH_H (0.03%) showed significantly more fat aggregates >4 μm (%) than the other ice creams, which did not show significant differences from the control ice cream (Table 3). Méndez-Velasco and Goff (2012) reported that fat-monoglyceride interaction in ice cream were altered by the degree of saturation of the monoglyceride. Changes in fat-emulsifier interactions influence ice cream quality by modifying the size and quantity of fat aggregates.
The effect of MPL+CH on protein displacement after ice cream production was determined. DATEM displayed the highest protein displacement (52%), and MPL (0.3%) alone showed the lowest protein displacement (13%; Table 3). Protein displacement increased by the use of MPL+CH combination. However, CH concentration in the combination had no significant effect on the protein displacement.
Emulsifiers used in ice cream promote protein displacement, especially casein micelles, from the fat-water interface and improve sensitivity to partial coalescence upon shearing (Goff, 2016). The air-water interface is not completely covered by partially-coalesced fat globules, and proteins adsorbed to the air-interface also play an important role in the aeration of ice cream (Zhang and Goff, 2004). According to a study of immunogold-labeled β-casein adsorption to the air interface in ice cream, the dissociation of casein micelles to soluble casein by EDTA improved protein adsorption at the air interface (Zhang and Goff, 2004). Therefore, in the context of ice cream production, CH might have a greater affinity for the air interface than casein micelles. Taken together, ice cream added with MPL+CH improved both protein displacement and partial coalescence when compared to MPL alone.
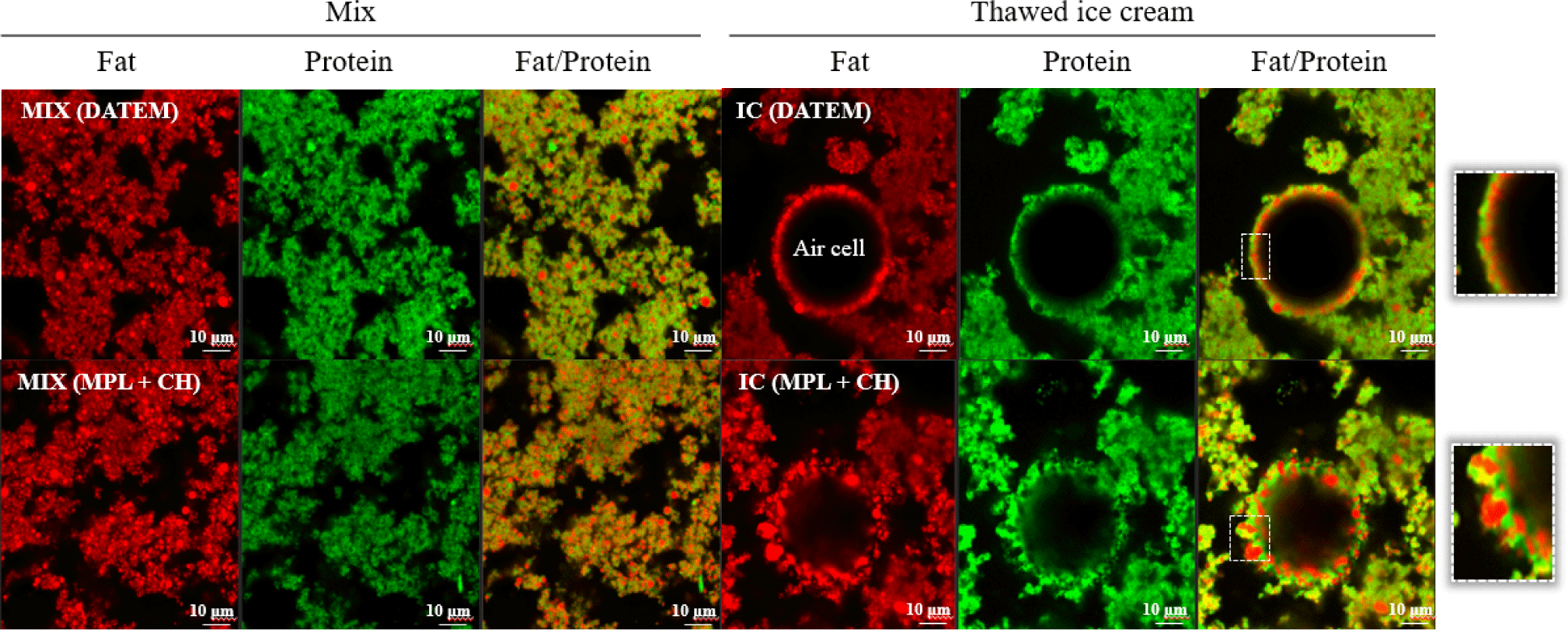
The structure of fat globules surrounding air cells in thawed ice cream was observed using CLSM. The representative image of control ice cream containing DATEM was compared with ice cream produced using a combination of MPL (0.3%)+CH (0.03%) because it showed comparable ice cream quality to the control ice cream. In the CLSM images, fat globules are stained red and the proteins are stained green (Fig. 5). There was no distinct difference among the ice cream mix samples, but there was some variation in the size of fat particles located on the air cell interfaces among the ice cream samples. Partially coalesced fat globules uniformly surrounded air cells in the control ice cream, whereas ice cream containing MPL+CH formed relatively heterogeneous larger-sized fat globules at the air cell interface. Although air cell surface coverage was not as uniform as the control ice cream, MPL+CH developed a thick layer of protein and coalesced fat on the air cell surface that might help enhance overrun. These results suggests that MPL+CH can be used as a potential emulsifier alternative to replace chemically synthesized emulsifiers such as DATEM.
Conclusion
The displacement of milk proteins from the emulsion droplet surface was significantly improved by the combination of MPL+CH compared to MPL alone. The increased milk protein displacement promoted partial coalescence of fat globules and increased ice cream overrun. The overall quality attributes of ice cream prepared using a combination of MPL+CH were comparable to those of the control ice cream. Therefore MPL+CH can be used as a healthy emulsifier in ice cream production.













