Introduction
Meat flavor is a multidimensional sensory attribute that substantially influences consumer preference and the overall perception of meat quality (de Araújo et al., 2022). It is shaped by a complex interplay between various volatile and non-volatile compounds formed during meat processing, cooking, and storage (Vilar et al., 2022). Key contributors to meat flavor include Maillard reaction products (MRPs), lipid oxidation products, and an array of amino acids, peptides, and nucleotides (Ramalingam et al., 2019; Sun et al., 2022). These compounds interact to create distinct aromas, tastes, and overall flavor characteristics that distinguish different types of meat and determine their acceptability to consumers (Dashdorj et al., 2015).
The Maillard reaction, which occurs between reducing sugars and amino acids during cooking, is particularly important for the development of characteristic browned flavors in cooked meat (Li et al., 2021). The products of this generate a myriad of volatile compounds, such as aldehydes, ketones, and pyrazines, which contribute to desirable roasted and savory notes (Mottram, 1998). In addition, lipid oxidation, which involves the breakdown of fats, results in the formation of compounds such as aldehydes, hydrocarbons, and alcohols, which add to the complexity of meat flavor (Fu et al., 2022).
Different cooking and aging methods, as well as meat processing techniques, can markedly affect meat flavor (Khan et al., 2015). Marination, curing, cooking, and smoking can also produce compounds that chemically impart a distinctive flavor (Smith and Acton, 2001). Even from the same carcass, meat cuts can substantially impact the formation of flavor compounds in meat (Wood, 2020), and this is influenced by the protein, fat, and moisture content of the meat, which in turn affects the flavor profile (Thu, 2006; Van Ba et al., 2012).
Recent advancements in analytical techniques, such as gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC), have greatly enhanced our ability to identify and quantify flavoring compounds (Bubli et al., 2021; Wei et al., 2019). These technologies allow the precise analysis of both volatile and non-volatile constituents of meat, providing detailed profiles of the flavor compounds present (Vilar et al., 2022). By understanding the composition and concentration of these compounds, researchers and industry professionals can improve meat quality and enhance consumer satisfaction.
Multi-omics approaches in meat flavor research integrate genomics, transcriptomics, proteomics, and metabolomics to comprehensively understand the molecular mechanisms underlying flavor formation. This holistic method enables the identification of key flavor-related genes, proteins, and metabolites, as well as their interactions and regulatory pathways. By providing insights into how factors like breed, diet, and processing influence flavor, multi-omics enhances precision in flavor optimization and supports targeted breeding and processing strategies.
The objective of this review was to effectively clarify meat flavor and contribute to the advancement of the meat industry based on a deeper understanding of meat flavor dynamics. Understanding the principles of various chemical reactions that contribute to meat flavor, along with the variables that differentiate meat flavors and molecular-level flavor component analysis techniques, can maximize the potential of meat’s flavor profile. In particular, unlike existing reviews that primarily focus on flavor-inducing components and their formation mechanisms at the molecular level derived from processing technologies (Xu et al., 2023) or the nutritional composition of meat (Fu et al., 2022; Khan et al., 2015), this review expands to an interpretation using holistic information about organisms, such as genome, transcriptome, proteome, lipidome, and metabolome, that may influence flavor. This comprehensive approach could provide bridge molecular insights with systems-level understanding, paving the way for innovative strategies to enhance meat flavor in both research and industry applications.
Flavor Chemistry in Meat and Meat Products
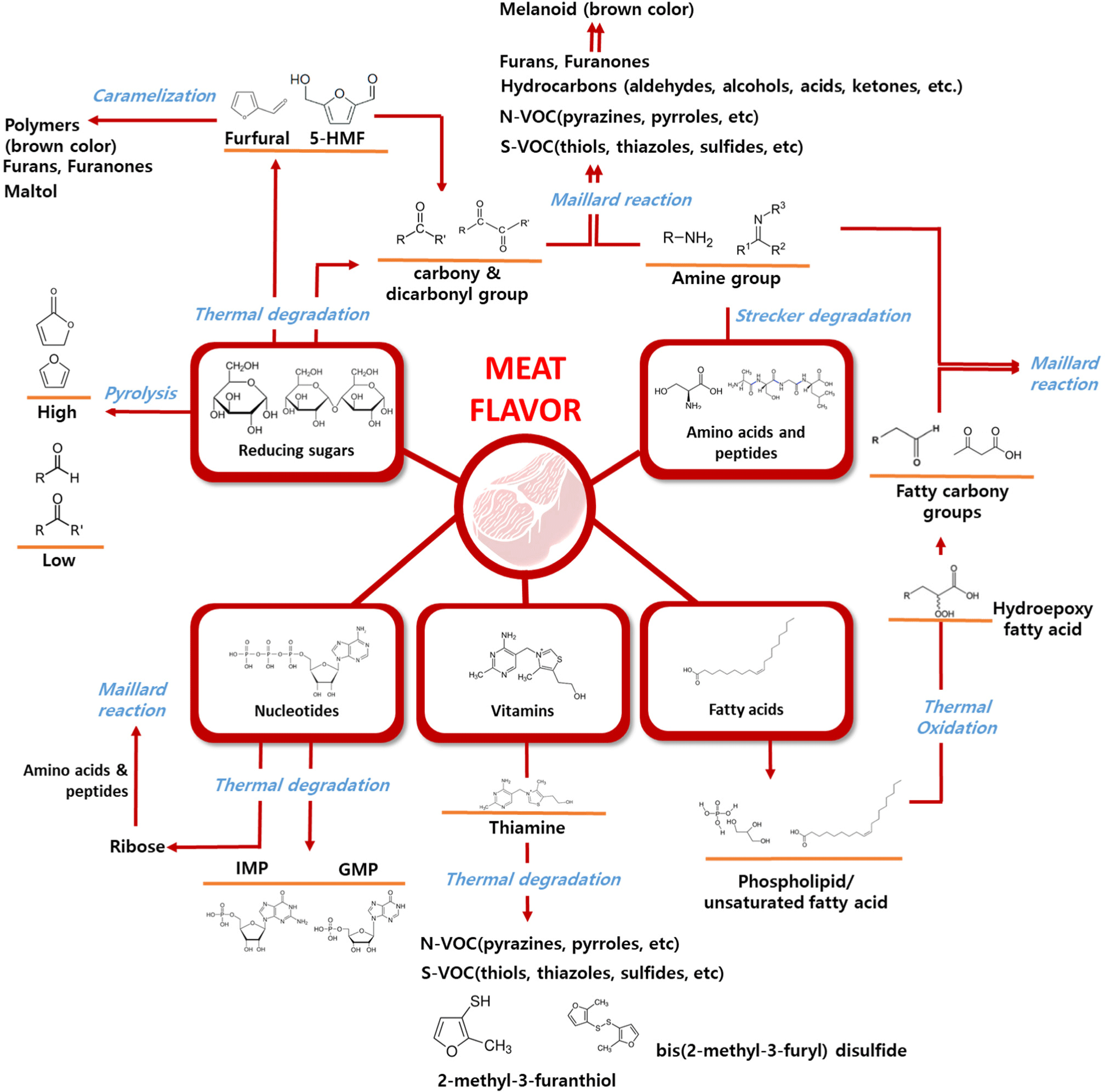
In general, raw meat has few flavor properties but a slightly smelly and blood-like taste (Van Ba et al., 2012), however, it contains abundant flavor precursors, including sugars, proteins, and lipids, which contribute to its meaty flavor. The well-recognized flavor of meat is primarily attributed to volatile compounds generated upon heat treatment. During heat processing, the abundant precursors in meat undergo various chemical reactions, breaking down into smaller molecules (Landy et al., 1996) or undergoing oxidation processes (Kanner, 1994), transforming into volatile compounds with low molecular weights and flavor properties. These molecular changes include several multiple reactions derived from heat treatment: the Maillard reaction, Strecker degradation, lipid degradation, and thiamine and ribonucleotide degradation. Fig. 1 illustrates the process of meat flavor formation, along with its precursors and the primary thermal reactions involved.
The Maillard reaction plays a crucial role in the formation of the unique flavor and color of meat. This nonenzymatic browning reaction occurs between reducing sugars and amino acids, producing various volatile flavor compounds that are essential to the sensory properties of cooked meat. Diverse volatile compounds, such as aldehydes, alcohols, ketones, furans, and their derivatives, including pyrrole, pyridine, pyrazine, thiophene, and sulfides, are generated during thermal processing (Chen et al., 2019; Sun et al., 2021; Yu et al., 2020). The Maillard reaction is not limited to simple sugars and amino acids and can also involve peptides. The involvement of food-derived peptides in the Maillard reaction produces MRPs that enhance meat flavor. Peptides with molecular weights ranging from 1,000 to 5,000 Da induce flavor-enhancing effects through the Maillard reaction (Fu et al., 2020). These MRPs are crucial for generating the umami taste and contribute to the overall palatability of meat (Kang et al., 2019).
Notably, the colors, yields, and types of flavor compounds produced are determined by the specific conditions under which the Maillard reaction occurs, such as temperature, pH, and humidity (Ribeiro et al., 2024; Starowicz and Zieliński, 2019; Wei et al., 2019). The reaction proceeds through three stages: the formation of early glycation products, their degradation, and the generation of flavor compounds such as pyrazines and thiophenes, which contribute heavily to the characteristic meat flavor (Starowicz and Zieliński, 2019). The distinctive meat flavor produced by the Maillard reaction is influenced by the presence of specific precursors such as cysteine, ribose, and lysine, which produce sulfur- and nitrogen-containing compounds (Raza et al., 2020; Wang et al., 2012b; Zhang et al., 2023b; Zhu et al., 2018). Glutathione, a tripeptide containing glutamate, cysteine, and glycine, forms sulfur-containing volatile compounds by cleaving peptide bonds during thermal processing. It generates important precursors of meat flavors, such as pyroglutamic acid and cyclic dipeptides (Wang et al., 2012b). The Maillard reaction is closely linked to lipid oxidation in cooked meat (Liu et al., 2024a; Mottram and Elmore, 2005; Zamora and Hidalgo, 2011). The interaction between these two processes enhances the complexity of the meat flavor profile because volatile compounds derived from lipid oxidation react with Maillard reaction intermediates. This generates heterocyclic compounds containing nitrogen and sulfur, which are important for the formation of cooked meat aroma.
The Maillard reaction also influences the color of cooked meat. The final stage of the reaction leads to the formation of melanoidins, brown polymers that markedly influence the visual appeal of meat products (Murata, 2021). The dark brown color associated with these polymers is often perceived as an indicator of a more intense flavor, making it a desirable quality for many meat products. Higher temperatures generally accelerate the reaction, leading to more intense flavor formation and darker color in the meat (Bekhit et al., 2019; Kong and Singh, 2016). In addition to flavor and color, the Maillard reaction also impacts meat texture (Starowicz and Zieliński, 2019; Sun et al., 2010). Cross-linking of proteins and other compounds during the reaction can influence meat tenderness, with high levels of MRPs contributing to a firmer texture (Renzone et al., 2022; Sulaiman et al., 2022).
Carbohydrate degradation plays a pivotal role in the formation of flavor compounds in meat. During the cooking process, carbohydrates, including pentoses and hexoses, are degraded through thermal reactions, such as the Maillard reaction, caramelization, and pyrolysis. Caramelization occurs at high temperatures (above melting temperatures of sugars) and converts sugars into compounds such as furfural and hydroxymethylfurfural, which are key contributors to flavor formation (Suleman et al., 2020). These intermediates can be further broken down into aromatic compounds, such as furans, which impart characteristic meat-like aromas. Chang et al. (2021) proposed a method for improving chicken flavor using a sugar-smoking technique. The authors primarily detected a notable increase in compounds such as furfural, 5-methyl-2-furancarboxaldehyde, 2-acetyl-5-methylfuran, and 1-(2-furanyl)-ethanone, known products of caramelization and the Maillard reaction. Pyrolysis also occurs upon exposure to high temperatures, leading to the production of various volatile compounds such as alcohols, aldehydes, and hydrocarbons. Sugars, particularly riboses from nucleotides, undergo degradation to form 5-methyl-4-hydroxyfuranone, a compound with a robust meat flavor (Begum et al., 2019). This process also releases hydrogen sulfide, which reacts with other flavor precursors to enhance the meat-like aroma (Shibamoto and Russell, 1976). The interaction between sugar degradation and the Maillard reaction, in which sugars react with amino acids, is another key pathway in the production of complex flavor compounds. This reaction generates volatile sulfur compounds such as thiophenes and thiazoles, which contribute to the toasted and roasted flavors typical of cooked meat (Shibamoto and Russell, 1976). In summary, the combination of carbohydrate degradation, the Maillard reaction, and the breakdown of other precursors, such as amino acids, thiamine, and nucleotides, results in a diverse and complex flavor profile associated with meat.
Strecker degradation is a chemical reaction that occurs when amino acids are degraded in the presence of dicarbonyl compounds produced during the Maillard reaction. This results in the formation of Strecker aldehydes, which are key contributors to the aroma of cooked meat (Chen et al., 2024). The interaction of Strecker degradation products with Maillard reaction intermediates leads to the formation of heterocyclic compounds, such as pyrazines, which further enhance the roasted flavor profile of cooked meat (Sohail et al., 2022). These interactions are crucial for generating the complex flavors associated with high-temperature cooking. Strecker degradation also produces sulfur-containing compounds, such as methanethiol and dimethyl disulfide, which are formed through the breakdown of methionine (Cerny, 2015; Schutte and Teranishi, 1974). Strecker degradation of sulfur-containing amino acids, such as cysteine and methionine, is particularly important in the formation of meaty and roasted flavors. These reactions produce volatile sulfur compounds that have a low odor threshold and strongly influence the aroma profile of cooked meat. Aldehydes generated from branched-chain amino acids, such as 2-methylbutanal and 3-methylbutanal, contribute to fruity and malty notes and add complexity to the flavor of meat (Wojtasik-Kalinowska et al., 2024). Strecker degradation is closely linked to lipid oxidation in meat. Lipid oxidation products can interact with Strecker intermediates, enhancing the development of flavor compounds such as aldehydes, which contribute to the overall sensory experience of cooked meat (Roldan et al., 2014; Utama et al., 2018).
In meat, fatty acids undergo oxidation, particularly at high temperatures, resulting in the formation of various volatile flavor compounds, such as aldehydes, ketones, and alcohols, which contribute to the overall flavor profile of cooked meat. During the heating process, phospholipids and triglycerides in meat undergo degradation, releasing short-chain fatty acids (Ren et al., 2024). These fatty acids are further oxidized to produce hydroperoxides, which can decompose into volatile compounds and enhance the aroma of cooked meat (Rasinska et al., 2019). The interaction between lipid oxidation products and MRPs is also essential for generating the complex flavor of cooked meat. Phospholipids play a pivotal role in these interactions, contributing to the formation of volatile compounds crucial for meat flavor (Cheng et al., 2024; Mottram and Edwards, 1983). Unsaturated fatty acids are particularly prone to oxidation, leading to the production of volatile compounds that can either enhance or degrade meat flavor depending on the degree of oxidation. Linoleic acid (C18:2n-6) and arachidonic acid (C20:4n-6) are easily oxidized, leading to the formation of volatile compounds, such as hexanal and 1-octen-3-ol, which are considered off-flavors in meat products (Yu et al., 2024).
To preserve meat quality, it is necessary to control lipid oxidation, which includes the use of antioxidants (Smet et al., 2008) and the maintenance of low temperatures (Soyer et al., 2010) during storage and cooking. Among the methods to inhibit lipid oxidation, freezing is effective in delaying irreversible biochemical reaction in meat, such as phospholipid oxidation and the generation of toxic compounds including malondialdehyde and cholesterol oxidation products (Soyer et al., 2010). These strategies help preserve meat flavor and extend its shelf life.
Thiamine (vitamin B1) is a bicyclic structure containing sulfur and nitrogen atoms, producing sulfur or nitrogen-containing heterocyclic compounds, such as furans, pyrimidines, thiols, thiazoles, sulfides, and disulfides (Brehm et al., 2019; Dwivedi and Arnold, 1973; Grosch, 2001). One important product is 4-methyl-5-(2-hydroxyethyl)thiazole, which is further degraded into various types of thiazoles. Specifically, it forms compounds such as 2-methyl-3-furanthiol and bis(2-methyl-3-furyl) disulfide, both of which are associated with strong meat flavors (Tang et al., 2013). Additionally, 2-acetylthiophene (toasty) and 2-formyl-5-methylthiophene (meaty) contribute to the flavor complexity of cooked meat (Feng et al., 2018). These volatile compounds derived from thiamine degradation were selected as representative compounds of the characteristic aroma of pork, which is rich in thiamine precursors (Han et al., 2021).
Nucleotide degradation, particularly of inosine monophosphate (IMP), is a key contributor to meat flavor, imparting characteristic umami and brothy flavors to meats such as pork and chicken (Yang et al., 2020; Zhang et al., 2018). The process through which the nucleotide adenosine monophosphate is broken down into IMP and hypoxanthine is responsible for changes in flavor during meat maturation, changing from a savory to a slightly bitter flavor with increasing hypoxanthine levels (Ichimura et al., 2017). The increase of hypoxanthine content (under 7.0 μmol/g in sample) has been reported to positively influence the taste of cured meat (Ichimura et al., 2017). The Maillard reaction, which involves ribose and amino acids from nucleotide degradation, also plays a role in producing sulfur-containing flavor compounds that contribute to the meaty aroma. The addition of IMP to beef at ten times its natural concentration increased the production of thiols and disulfides containing furan groups, which are key compounds that contribute to the aroma of meat (Ichimura et al., 2017).
Factors Influencing the Flavor of Meat
Meat flavor, a representative indicator of meat quality, is affected by animal species, sex, age, feed, and processing. The effects of these factors on meat flavor are described in Table 1. Animal species possess unique flavors owing to differences in their carcass composition, including protein, fat, and moisture. Meat flavor can be influenced by factors such as total fat, intramuscular fat, and fatty acid composition. Fat levels impact the fatty acid profile of meat, as greater fat accumulation tends to increase the amounts of saturated and monounsaturated fatty acids more rapidly, thereby reducing the relative proportions of polyunsaturated fatty acids and the polyunsaturated/saturated ratio (De Smet et al., 2004). Van Ba et al. (2013) concluded that the breed (e.g., Hanwoo versus Angus) could considerably impact the physicochemical quality, sensory characteristics, and content of volatile flavor compounds in meat.
| Factors | Influencing meat flavor | References |
|---|---|---|
| Animal species | Animal species have a unique flavor due to differences in protein and fat content. | Dinh et al. (2021); Park et al. (2024) |
| Animal sex | Differences in intracutaneous and subcutaneous fat between the sexes of animals may influence flavor-related compounds. | Jayasena et al. (2014); Khan et al. (2015); Lorenzo et al. (2013) |
| Animal age | Animal age affects intramuscular collagen solubility and fatty acid composition, affecting flavor. | Jaborek et al. (2020) |
| Animal feed | Animal feed affects flavor by changing the fatty acid composition and meat composition. | Wood et al. (2008) |
| Processing (curing, aging, cooking, and smoking) | Curing produces desirable flavors by chemical reactions of proteins and fats with salt and nitrite. | Martin, (2001); Ramarathnam and Rubin (1994) |
| Aging enhances the flavor of meat by increasing the amount of flavor compounds via enzymatic action or by increasing the amount of volatile compounds derived from fatty acid degradation. | Dou et al. (2022); Liu et al. (2024a) | |
| Cooking enhances the flavor of meat by impacting the amount of free amino acids, carnosine, pyrazine, and hexanol. Cooking also induces the oxidation of lipids contained in meat to form flavor. | Khan et al. (2015); Lorenzen et al. (2005); Zhu et al. (2019) | |
| Smoking affects the satisfactory flavor of meat owing to the influence of phenols, alcohols, methyl ketones, and esters. | Guo et al. (2021); Van Ba et al. (2012) |
Additionally, the impact of animal sex on meat flavor has been well documented. Gorraiz et al. (2002) reported that Pirenaica and Friesian bulls and heifers demonstrated notable differences in volatile compounds, odors, and flavors. After cooking, bull beef had a bloody flavor that was linked to a high 2-propanone content, along with a more pronounced liver-like odor and flavor, whereas heifer beef exhibited a robust characteristic flavor. The differences in juiciness and flavor intensity between male and female lambs could be attributed to variations in intramuscular fat, which plays a major role in the development of aroma and flavor in meat (Brennand and Lindsay, 1992).
The age of an animal can markedly impact flavor, resulting in a distinctive aroma or poor quality as it ages (Fry et al., 1958). Khan et al. (2015) reported that age impacts collagen solubility in the muscle and increases flavor intensity, with older animals possessing higher levels of straight-chain fatty acids. Foraker et al. (2020) found that animal age affects flavor and also influences overall taste in meat quality.
Animal feed is a notable cost factor in livestock production, and the type of feed plays a crucial role in determining carcass conformation and the physicochemical and organoleptic characteristics of meat, such as proximate composition, fatty acid profile, tenderness, and color (Andersen et al., 2005; Dinh et al., 2021; Wood et al., 2008). Feed systems affect carcass composition and fattening, which can affect meat flavor (Watkins et al., 2013). Young et al. (1997) reported that feed type affected the fatty acid composition of meat. Additionally, the authors detected the presence of volatile compounds such as terpenes and diterpenoids in pasture-raised lambs. Grain-finished animals also possess high concentrations of short-branched-chain fatty acids, which are associated with the “mutton” aroma of cooked sheep meat (Young et al., 2003). According to Melton (1990), high-energy grain diets produced more acceptable or more intense flavors in red meat than low-energy forage or grass diets, and that flavor changes were greater in beef than in pork as unsaturation in the diet increased.
The meat processing process can also impart a unique flavor, and various technologies have been developed to enhance this flavor. Curing, aging, cooking, and smoking are used to impart or enhance meat flavor. Wang et al. (2012a) showed that decreasing the level of curing salt increased the formation of flavor-active volatiles in dry-cured turkey ham. Jia et al. (2024) reported that the addition of salt and nitrates/nitrites for meat curing was associated with the color and flavor of cured meat. Dou et al. (2022) reported that aging can improve meat flavor by increasing the amount of flavor compounds through enzymatic action, thereby enhancing the amount of volatile compounds. Liu et al. (2024b) detected the presence of 62 volatile flavor compounds during the dry-aging period, and the contents of Strecker aldehydes (2-methyl-butanal and 3-methyl-butanal), acids, heterocyclic compounds, and ethyl acetate increased with increasing dry-aging time. Zhu et al. (2019) reported that cooking enhances the flavor of meat owing to its effects on the amounts of free amino acids, carnosine, pyrazine, and hexanol. Guo et al. (2021) found that wood-smoked bacon had stronger smoke and fat aromas than liquid-smoked bacon, and aldehydes were the most abundant compound groups. Begic et al. (2022) detected a positive correlation between the contents of phenols and hydrocarbons, alcohols, ketones, esters and lactones, terpenes, aromatic hydrocarbons, and acids in dry-smoked goat meat using principal component analysis. Therefore, the meat processing process can increase the amount of volatile compounds to impart a unique flavor to meat, and the mechanism for producing flavor components can also change depending on the processing process method.
Techniques for Identifying Flavor Compounds in Meat
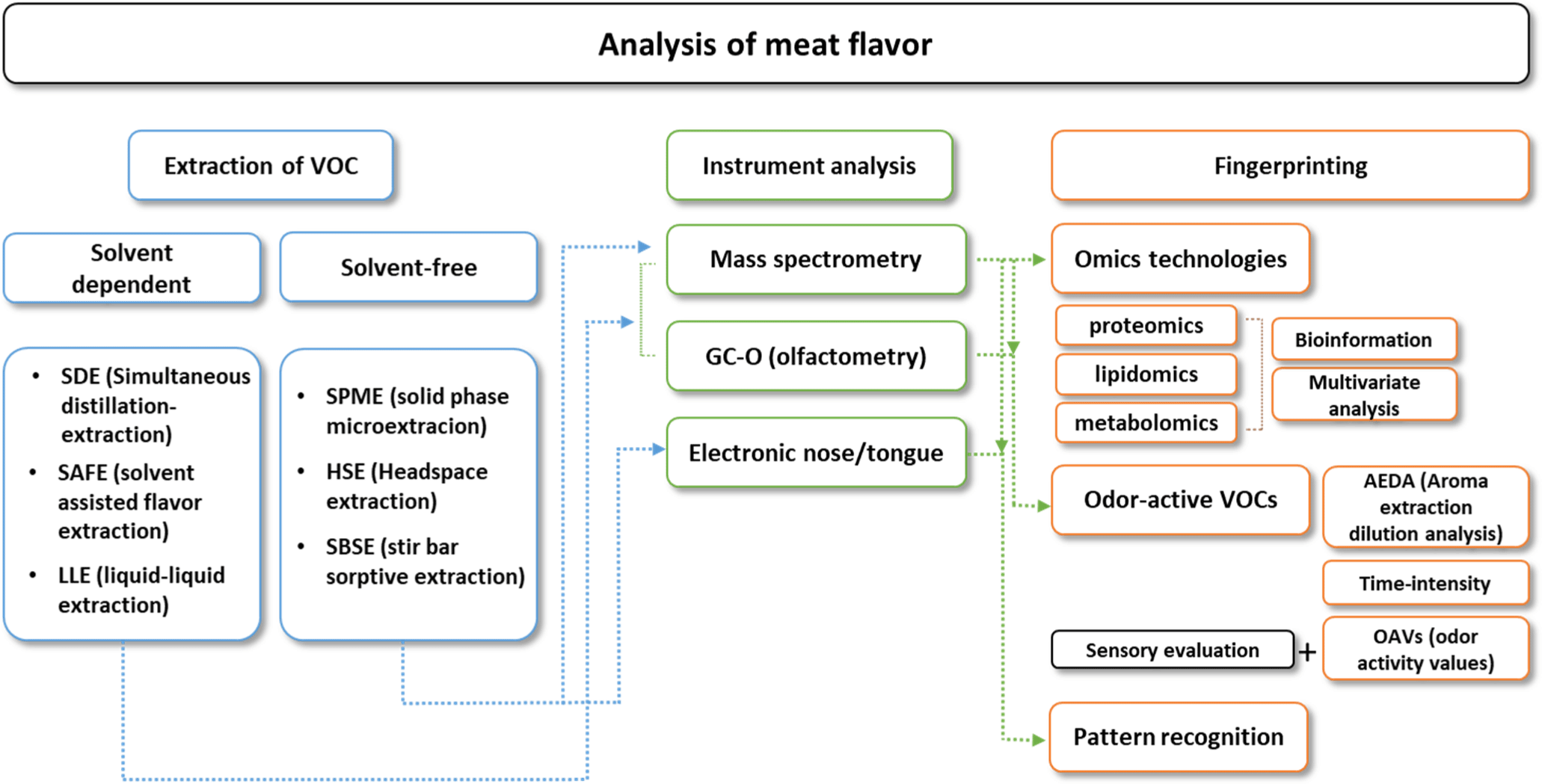
The components that contribute to the flavor and aroma of meat are highly dynamic and diverse, making it advisable to apply strategies and techniques tailored to the specific research objectives (Fig. 2). Volatile compounds are organic chemicals that easily evaporate and release distinct odors, which are commonly found in plants, fruits, and essential oils. These compounds play crucial roles in determining the aroma and flavor profiles of foods, beverages, and perfumes (Chambers and Koppel, 2013; Guichard, 2002). They are highly reactive and unstable; therefore, various factors, such as temperature, extraction time, and interaction with the matrix, must be considered when extracting volatile compounds from food (Madruga et al., 2009; Wagner and Franco, 2012). Food flavor analysis is typically performed to separate individual volatile compounds from the food matrix based on their physicochemical properties while simultaneously gathering the entire set of volatile compounds. It can efficiently extract volatile compounds from foods using the chemical properties of solvents; however, it has high selectivity owing to the affinity between the compounds and the solvent. Recently, there has been an increasing preference for extraction methods that avoid the use of organic solvents or employ nonthermal techniques.
Steam distillation is a typical extraction process, in which steam is used to separate volatile compounds from a liquid matrix at low temperatures. Simultaneous distillation extraction combines steam and solvent extraction and is an important extraction method for isolating 3-methylindole and p-cresol, which are crucial to the ‘pastoral’ flavor characteristics of lamb (Schreurs et al., 2007). Solvent-assisted flavor evaporation is a gentle technique that operates at low temperatures and high vacuum, preserving sensitive compounds during extraction. It can be used for flavor extraction from milk, raw meat, and ham (Colahan-Sederstrom and Peterson, 2005; Liu et al., 2022); however, it is a time-consuming and laborious process. Solid-phase microextraction (SPME) involves exposing a fiber coated with an adsorbent to the sample or headspace to capture volatile compounds. This technique is preferred for flavor analysis owing to its simple extraction process, cost-effectiveness, solvent-free nature, and high sensitivity. SPME is an effective extraction method capable of identifying volatile compounds in various meat products, as demonstrated in a previous study that compared volatile compound extraction from beef and lamb fats (Watkins et al., 2012). Stir-bar sorptive extraction (SBSE) uses a coated stir bar to absorb volatile compounds from a sample in the absence of solvents. SBSE has an extraction phase that is 50–250 times larger than that of SPME (Ngamchuachit et al., 2020), resulting in enhanced extraction capabilities, and is used to extract volatile compounds from various meat products (Benet et al., 2015; Ngamchuachit et al., 2020).
Mass spectrometry (MS) is a typical analytical method for volatile flavor compound analysis owing to its ability to provide detailed chemical identification and quantification of individual compounds. Coupled with GC-MS, MS facilitates the precise separation and detection of complex volatile compound mixtures. Liquid chromatography-MS (LC-MS) is well suited for in-depth flavor analysis, enabling the identification of semi- or non-volatile compound profiles in foods. To identify key flavor compounds and elucidate the mechanism of flavor formation in foods, it is important to identify the composition of volatile and non-volatile compounds. Kang et al. (2024) investigated key volatile and non-volatile metabolites and their metabolic pathways using GC-MS and LC-MS to determine the molecular regulatory mechanisms and support molecular breeding for yak meat flavor formation. In addition, Zhang et al. (2025) provided a rapid LC-MS/MS method to detect livestock species in meat products.
The electronic nose (E-nose) mimics human olfactory senses by using an array of sensors to detect volatile compounds based on patterns, although it cannot identify individual compounds. It is used for the rapid classification and comparison of aroma profiles, particularly for quality control and differentiation of products. Similarly, the electronic tongue (E-tongue) assesses flavor attributes, such as sweetness, bitterness, and umami, using sensors that detect taste-related chemicals in liquids. These electronic sensory devices can be used to determine the freshness of meat samples (Chotimah et al., 2024), provide indicators to distinguish their origin (Yuan et al., 2024), or yield information regarding unique flavor characteristics depending on the muscle type (Zhou et al., 2024).
Gas chromatography-olfactometry (GC-O) combines chemical analysis with sensory evaluation; human panelists sniff the GC effluent to identify odor-active compounds. GC-O is particularly useful for detecting specific compounds that contribute the most to the overall aroma or flavor of a product. Determination of odor-active compounds involves sensory and chemical techniques, including aroma extract dilution analysis (AEDA) and time-intensity methods. In AEDA, flavor extracts are diluted in a stepwise manner, and panelists evaluate each dilution for aroma, allowing identification of the most potent odor-active compounds based on their detection at higher dilutions. The time-intensity method records the perception of an aroma over time, providing insight into the duration and intensity of the impact of an odor-active compound. To quantify the contribution of these compounds, the odor activity value (OAV) is calculated by dividing the concentration of each compound by its odor threshold (the concentration at which it can be perceived). Compounds with an OAV greater than one are considered to substantially contribute to the overall aroma. The identification of odor-active compounds in meat not only provides crucial indicators for the sensory perception of meat flavor but also provides essential information for recreating its aroma (Nie et al., 2024; Pu et al., 2020; Wang et al., 2022). These methods allow researchers to prioritize compounds not only by presence but also by their sensory relevance, combining chemical data with human perception to effectively identify the compounds that define a product’s aroma profile. This approach ensures that both potency and perceptibility are considered in flavor analysis.
Recent advances in high-throughput sequencing technologies, particularly genomics, transcriptomics, proteomics, and metabolomics, have enabled a comprehensive understanding of meat quality and taste at the molecular level (Table 2). These omics technologies are employed to explore the genetic, protein, and metabolic contributions to meat quality. Genomic studies have focused on identifying genes related to economically important traits such as tenderness, fat deposition, and meat color (Arikawa et al., 2024; Marín-Garzón et al., 2021). Advanced sequencing tools, such as long-read sequencing (Liu et al., 2024c) and combination with artificial ingelligence (Hamadani et al., 2022), have made it easier to investigate the role of these genes in improving meat quality. Long-read sequencing is a technology developed to overcome the limitations of NGS (Next-Generation Sequencing), which struggles with errors in genome assembly and difficulties in decoding repetitive regions due to its short read lengths. It leverages the advantages of TGS (Third-Generation Sequencing), including long read lengths, real-time base sequencing, and the ability to directly sequence DNA/RNA without PCR amplification. TGS offers significant advantages in livestock research by enabling the detection of rare genes, structural variations, and transcriptional complexity, while facilitating superior breeding, genetic reproduction, and epigenetic analysis through its long reads, real-time sequencing, and reduced bioinformatics costs.
| Keywords | Samples | Extraction | Instrument | Analytical method | References |
|---|---|---|---|---|---|
| Flavor extraction | Chicken, pork, duck, beef, mutton meat | HS-SPME | GC-MS | Volatile profiling Multivariate analysis (PCA, PLS_DA, OPLS-DA) | Yu et al. (2025) |
| Beef, raw ham, baked ham, pork sausage, and chicken meat | HS-SPME | GC-MS | Volatile profiling Multivariate analysis (PCA) | Acquaticci et al. (2024) | |
| Dry-cured ham | Headspace sorptive extraction, DHE | GC-MS | Volatile profiling Multivariate analysis (PCA) | Segura-Borrego et al. (2023) | |
| Grilled chicken | DHE, SBSE | GC-O/MS | Volatile profiling, olfactory detection | Ngamchuachit et al. (2020) | |
| Dry-rendered beef fat | SAFE | GC-MS, GC-O | Odor-active compounds identifying | Yang et al. (2023b) | |
| Jinhua ham | SAFE, SPME, needle trap | GC–TOF/MS | Odor-active compounds identifying | Liu et al. (2022) | |
| Electronic sensor | Vacuum-packaged chicken meat | Headspace extraction | Electronic nose | Discrimination of freshness | Chotimah et al. (2024) |
| Yak jerky | Headspace extraction, aqueous solution | Electronic nose/tongue, GC-IMS | Flavor profiling and discrimination model | Zhou et al. (2024) | |
| Sauced pork | Headspace extraction, aqueous solution | Electronic nose/tongue, GC-MS, GC-IMS | Discrimination of different regions | Yuan et al. (2024) | |
| Omics technologies | Beef cattle | - | - | Genomics | Arikawa et al. (2024) |
| Pork meat | - | - | Transcriptomics | Wang et al. (2024) | |
| Broiler chicken breast | 2% SDS buffer | HPLC-MS/MS | Proteomics | Yang et al. (2023a) | |
| Beef meat | Acid hydrolysis, SE (cold acetone) | LC-MS/MS | Proteomics | Kim et al. (2021) | |
| Chicken meat | LLE (chloroform/methanol) | UPLC-ESI-MS | Lipidomics | Zhang et al. (2023a) | |
| Dry-cured mutton ham | Solvent extraction | UPLC-MS/MS | Lipidomics | Guo et al. (2022) | |
| Meat exudate | LLE (cold methanol/water) | UHPLC-MS/MS | Metabolomics | Yu et al. (2024) | |
| Goat meat | LLE (methanol/acetonitrile/water) | UHPLC-Q-Orbitrap-MS | Metabolomics | Jia et al. (2021) | |
| Aged pork | LLE (methanol/chloroform/water) | GC-TOF/MS | Metabolomics | Tamura et al. (2022) | |
| Roasted quail meat | SAFE, LLE (chloroform/methanol) | GC-O/MS, HPLC-HRMS | Lipidomics and volatilomics | Liu et al. (2024a) | |
| Pork cuts | Headspace extraction, LLE (chloroform/methanol) | GC×GC-TOF/MS, LC-MS/MS | VOC, lipidomics and transcriptome | Zhang et al. (2025) | |
| Xinjiang brown beef | Headspace extraction, SE (methanol) | GC×GC-TOF/MS, LC-MS | Metabolomics and transcriptomics | Ma et al. (2024) | |
| Sensory test | Chicken broth | HS-SPME, SAFE | GC-O/MS | AEDA | Nie et al. (2024) |
| Beef flavoring | SPME, DHS, LLE | SGC×GC-O/MS, sensory test | AEDA, OAV | Wang et al. (2022) | |
| Smoked cured pork meat | SDE | GC-MS, GC-O/MS, sensory test | AEDA, OAV | Pu et al. (2020) |
HS-SPME, headspace-solid phase microextraction; DHE, dynamic headspace extraction; SBSE, stir bar sorptive extraction; SAFE, solvent-assisted flavor extraction; SE, solvent extraction; LLE, liquid-liquid extraction; SDE, simultaneous distillation and extraction; GC-MS, gas chromatography-mass spectrometry; GC-O, gas chromatography-olfactometry; GC-TOF/MS, gas chromatography-time of flight-mass spectrometry; GC-IMS, gas chromatography-ion mobility spectrometry; LC-MS, liquid chromatography-mass spectrometry; UPLC-ESI-MS, ultra-performance liquid chromatography-electrospray ionization-mass spectrometry; SGC×GC-O/MS, switchable two-dimensional gas chromatography-olfactometry-mass spectrometry; PCA, principal component analysis; PLS-DA, partial least squares-discriminant analysis; OPLS-DA, orthogonal partial least squares discriminant analysis; AEDA, aroma extract dilution analysis; OAV, odor activity values.
Research on meat flavor using transcriptome analysis contributes to improving the taste and quality of meat by uncovering various gene expression patterns and metabolic pathways. Transcriptomics, which focuses on gene expression profiles, has revealed the influence of genes on fat accumulation, muscle development, and tenderness (Bongiorni et al., 2016; Wang et al., 2024). These data help us understand how feeding management and other factors affect meat quality at the genetic level. Studies using transcriptomic approaches to investigate the molecular basis of beef quality highlight the strengths of high-throughput transcriptomics as a more sensitive and accurate analytical method for comprehensively exploring the transcriptional landscape of biological systems (Wang et al., 2009).
Molecular-level research, including proteomics, lipidomics, and metabolomics, can integrate data obtained from various biological layers, advancing meat quality and flavor studies in a more precise and practical direction. Proteomics is a technology that analyzes protein expression, modifications (e.g., phosphorylation, glycosylation), and interactions to identify their functional roles, making it highly useful for meat quality analysis. Proteomics is used to investigate how proteins influence meat quality, particularly how they change during the pre- and post-slaughter phases (Kim et al., 2021; Lamri et al., 2023). Proteomics has also revealed the impact of various factors, such as animal breed, on protein expression profiles in meat (Luca et al., 2022). Lipidomics comprehensively analyzes lipid metabolites, including studies on fatty acid composition and lipid metabolic pathways. It is particularly important for evaluating fat accumulation and its impact on meat flavor and juiciness (Guo et al., 2022; Zhang et al., 2023a). Lipid metabolism plays a pivotal role in determining the organoleptic properties of meat, including its taste and texture (Ramalingam et al., 2019). Finally, metabolomics aims to quantify and qualitatively analyze the end products of metabolism to interpret biochemical pathways. Metabolomics focuses on small molecules and metabolites involved in real-time changes in meat quality. Based on the metabolic approach, biochemical processes such as lipid oxidation and glycolysis contribute to the flavor and tenderness of meat (Jia et al., 2021; Tamura et al., 2022; Yu et al., 2024).
Multi-omics approaches integrate different omics platforms to provide deeper insights into the molecular mechanisms responsible for meat quality. This integration facilitates the identification of biomarkers related to taste, texture, and other sensory attributes. Multi-omics approaches have also been applied to study food fraud and the authenticity of meat products. Comprehensive strategies are particularly useful for detecting species-specific markers that can reveal the adulteration of meat products (Liu et al., 2024d; Ma et al., 2024; Zhang et al., 2025). Proteomics and metabolomics can be to uncover regulatory connections between proteins and metabolites, while metabolomics and transcriptomics has primarily explored how fat deposition can be controlled and tenderness of meat is enhanced though the involvement of various genes and signaling pathways. According to research on beef quality (Ma et al., 2024), intramuscular fat is a key factor in determining beef quality. Through integrated omics approach, including metabolomics and trasncriptomics, it was revealed that the composition of flavor compounds significantly differed based on the contents of intramuscular fat and identified major genes associated with this variation. In meat science, omics research involves improving the accuracy and efficiency of these technologies, which includes optimizing bioinformatic tools and expanding existing databases to better predict and control meat quality.
Conclusion
This review provides insights into effective strategies for understanding meat flavors. The strategic presentation of meat flavor, the most important factor affecting meat quality, could be a cornerstone in advancing the meat industry. Meat flavor is primarily developed through heat-induced chemical reactions that transform flavor precursors such as sugars, proteins, and lipids into volatile compounds. These reactions, including the Maillard reaction and lipid degradation, contribute to the complex flavor profile of cooked meat. Meat flavor is influenced by factors such as species, sex, age, feed, and processing, which affect its physicochemical, sensory, and volatile compound characteristics. Processing methods like curing, aging, cooking, and smoking enhance flavor by altering volatile compounds and flavor precursors. Volatile compounds, essential for aroma and flavor, are extracted using methods like steam distillation, SPME, and SBSE, with analysis often conducted via GC-MS or LC-MS for detailed profiling. Advanced techniques, including electronic noses and GC-O, integrate sensory and chemical data to identify key odor-active compounds and their contributions to food flavors. Omic technologies, including genomics, proteomics, and metabolomics, offer comprehensive insights into the molecular mechanisms that influence meat quality, such as flavor, tenderness, and fat composition. This review was aimed at overcoming this situation and providing insights into the development of the meat industry, thereby contributing to its development of the meat industry.