Introduction
Poultry farming is vital to the worldwide livestock sector, providing a substantial source of protein and nutrition for growing humans population (Mottet and Tempio, 2017). The demand for poultry products has been gradually increasing due to numerous factors including population increase, rising income levels, and evolving dietary preferences (Clonan et al., 2016; Saeed et al., 2019; Yatao et al., 2018). The poultry industry constitutes a significant segment of the global agricultural sector, especially in developing nations (Mnisi et al., 2023), where ever rising human population needs the introduction of alternative sources of dietary protein to avoid the hazards related to protein deficits (Mnisi et al., 2023). Poultry plays a crucial role in global food security by providing affordable, high-quality protein in the form of meat and eggs. Poultry is a vital source of meat and eggs containing high-quality proteins, enriched with essential amino acids, vitamins and minerals to fulfill the nutritional requirements, vital for addressing malnutrition and combat food security issues in low-income population (Gržinić et al., 2023; Lingala et al., 2024). Additionally, the contribution of poultry in food security extends beyond the nutritional requirements. It plays an important role to enhance the livelihood of low-income population, owing to the economically viable and short production life (Vlaicu et al., 2024). Nonetheless, the manufacturing of poultry products demonstrates several obstacles, including environmental degradation, elevated feed costs, and the dissemination of diseases (Mottet and Tempio, 2017). Another hurdle which increase of feed price is the extravagant feed ingredients, including grains, oilseeds, synthetic chemicals and feed supplements to raise the production cost (Arif et al., 2017; Safdar et al., 2024). To tackle these difficulties, there is increasing interest in identifying alternative feed ingredients that can improve the nutritional quality of poultry diets simultaneously reducing the adverse environmental effects of poultry production (Arain et al., 2022b; Nabi et al., 2020a; Nabi et al., 2020b; Saeed et al., 2018). Poultry feed researchers emphasize for the investigation of alternative feed ingredients, including novel protein sources, insect-derived feed, algae, and plant-based bioactive substances from the food and agricultural sectors, to substitute expensive ingredients and attain production objectives (Behan et al., 2024; Nabi et al., 2024).
Microalgae are photosynthetic microorganisms acknowledged as a viable alternative feed component in poultry diets owing to their elevated nutritional content and functional attributes (Koyande et al., 2019). Furthermore, microalgae represent a wide assemblage of organisms capable of thriving in various settings, including freshwater (Maltsev and Maltseva, 2021), marine water (Bhuvana et al., 2019), and waste water. They are an abundant source of protein and vital fatty acids (Sathasivam et al., 2019), vitamins (Wang et al., 2021), minerals (Uribe-Wandurraga et al., 2019), and source of antioxidants (Gauthier et al., 2020), all these constitute essential elements of a balanced poultry diet (Madeira et al., 2017). While, microalgae have demonstrated the ability to augment the growth and immune response of poultry, in addition to enhancing the quality of poultry products (El-Ghany, 2020). These microscopic photosynthetic organisms are a sustainable and renewable resource, containing higher concentration of essential nutrients, supporting the growth and productive performance of poultry. The lipid fraction, of microalgae particularly omega-3 fatty acids like docosahexaenoic acid (DHA), boost immunity and promoting gut health, thus enhances the productivity and nutritional quality of poultry based food such as meat and eggs (Kalia and Lei, 2022).
Present study provides a comprehensive overview of the current understanding on incorporation of microalgae in poultry diets. Moreover, current review offers a comprehensive analysis of the nutritional value, functional features, and advantageous applications of microalgae in poultry nutrition to improve the health and productivity of poultry. Simultaneously, the prospective advantages and obstacles related to the use of microalgae in poultry feed should also be emphasized.
Categories and Characteristics of Microalgae
Microalgae constitute a complex assemblage of photosynthetic microorganisms inhabiting a variety of aquatic habitats, encompassing both freshwater and marine ecosystems (Saber et al., 2022). They are essential to global ecological and biogeochemical cycles, greatly contributing to primary production and serving as the foundation of the food sources for aquatic animals (Lobus and Kulikovskiy, 2023; Quintas-Nunes et al., 2023). These unicellular or multicellular microbes may utilize both organic and inorganic carbon sources for growth and development, rendering them an optimal platform for large-scale biomass production (Chen et al., 2024). The diversity of microalgae exemplifies their flexibility and the extensive variety ranging from unicellular species like Chlorella to multicellular forms like Spirogyra. Their ability to adapt to diverse habitats, including extreme environmental settings (Singh et al., 2023).
The classification of microalgae is intricate owing to their extensive diversity and the ongoing identification of novel species. They are often categorized according to their pigmentation, cell wall composition, storage products, and various morphological and physiological characteristics (Shaikh et al., 2022). Microalgae are taxonomically categorized into numerous principal groups, including green algae, diatoms, cyanobacteria, and dinoflagellates, among others (de Morais et al., 2016; Masojídek and Torzillo, 2014). The distinct attributes of each category, including their pigment makeup, cellular architecture, and metabolic pathways, influence their distinctive qualities and prospective applications (Madkour et al., 2023; Masojídek and Torzillo, 2014).
Green algae are recognized for their capacity to a mass substantial lipid levels, rendering them a prospective source for biofuel production (Dolganyuk et al., 2020; Khan et al., 2018). Diatoms are acknowledged for their capabilities in wastewater treatment and the synthesis of key biochemical, such as carotenoids and silica (Quintas-Nunes et al., 2023). Cyanobacteria, sometimes referred as blue-green algae, have attained interest for their capacity to synthesize a diverse array of bioactive substances, such as pigments, vitamins, and antioxidants.
Nutritional Composition of Microalgae
Microalgae possess the capability to synthesize a diverse array of important substances, such as proteins, lipids, carbohydrates, vitamins, and pigments, which are utilized in the feed, medicinal, and biofuel sectors (Dolganyuk et al., 2020). The scientific investigation of microalgae has attracted heightened interest in recent years owing to its exceptional potential as a sustainable and nutrient-rich source of feed, and valuable bioactive chemicals (Bature et al., 2022).
The nutritional makeup of microalgae can vary considerably based on species, growth conditions, and post-harvest processing techniques (Alghazeer et al., 2022). Light intensity, temperature, nutrient availability, and growth strategies can all affect the accumulation of certain nutrients in microalgae cells. The fundamental advantage of microalgae is their remarkable nutritional profile. Microalgae are recognized for their abundance of vital elements, such as proteins, carbs, lipids, vitamins, and minerals (Sanjari et al., 2018). The energy content of microalgae biomass is determined by the existence of carbon, oxygen, hydrogen, nitrogen, and other components, rendering them a viable source of renewable energy. Moreover, microalgae have evolved intricate biochemical and physiological mechanisms to thrive in extremely competitive environments, resulting in the accumulation of important bioactive chemicals (Rendón-Castrillón et al., 2020). Microalgae can possess a remarkable protein concentration, with certain species up to 70% protein on a dry matter basis (Sanjari et al., 2018). These proteins are often of superior quality, encompassing all essential amino acids necessary to meet the nutritional requirements of animals. Moreover, microalgae serve as a substantial source of omega-3 and omega-6 fatty acids, essential for augmenting immune response and improving the health and productivity of animals. Specific microalgae species are recognized as superior producers of carotenoids, including astaxanthin and lutein, which have strong antioxidant capabilities and are associated with numerous health advantages (Novoveská et al., 2023).
Moreover, microalgae are recognized as a substantial source of lipids, especially triacylglycerol’s, which can be utilized for many industrial purposes (de Morais et al., 2016). The lipid content of microalgae is affected by several parameters, including species, growing circumstances, and nutrient availability (Rendón-Castrillón et al., 2020). Besides lipids, microalgae also store considerable quantities of carbohydrates, which can improve their nutritional utility in cattle feed (de Morais et al., 2016). Furthermore, microalgae are distinguished by their pigments, including carotenoids and phycobilipigments, which enhance their vivid coloration and exhibit antioxidant and therapeutic attributes. Moreover, microalgae serve as a superior supplier of important vitamins and minerals, encompassing vitamin A, B-complex vitamins, vitamin C, vitamin E, along with minerals such as iron, calcium, and magnesium. Likewise, Dineshkumar et al. (2017) documented the biochemical and elemental analysis of green algae composition was depicted in Table 1. The nutritional value of microalgae can be augmented by crop optimization and genetic engineering.
| Species of microalgae | Carbohydrates | Protein | Lipids | Total dietary fiber | References |
|---|---|---|---|---|---|
| Chlorella vulgaris | 20.99 | 15.67 | 41.51 | 15 | Alfaia et al. (2021); Chia et al. (2015) |
| Spirulina platensis | 30.21 | 13.3 | 48.36 | 14.98 | Seghiri et al. (2019) |
| Chlorella sorokiniana | 35.67 | 9.9 | 18.81 | 13.5 | Chia et al. (2015); Niccolai et al. (2019) |
| Nannochloropsis oceanica | 22.7 | 24.8 | 19.1 | 20 | Zanella and Vianello (2020) |
| Scenedesmus obliquus | 13.41 | 4.66 | 30.38 | 19.37 | Ferreira et al. (2020) |
| Dunaliella tertiolecta | 21.69 | 2.87 | 61.32 | 9.06 | Araj‐Shirvani et al. (2024) |
| Dunaliella salina | 32 | 57 | 9 | 48.82 | Shantkriti et al. (2023) |
| Scenedesmus dimorphus | 36.5 | 13 | 28 | 32.7 | Tapia-López et al. (2024) |
| Chlorococum humicola | 32.5 | - | - | 24.5 | Yang et al. (2022) |
| Chlamydomonas reinhardtii | 22.6 | 64.7 | 12.6 | 11.9 | Darwish et al. (2020) |
| Spirogyra sp. | 48.5 | 13 | 16 | 37 | Sriwattana et al. (2024) |
| Porphyridium cruentum | 48.5 | 33.5 | 11.5 | 21.7 | Castro‐Varela et al. (2021) |
| D. salina | 85.58 | 8.46 | 11.47 | 0.8 | Demirel (2022) |
| Arthrospira platensis | 42.08 | 14.95 | 6.5 | 42.82 | Markou et al. (2023) |
| Scenedesmus almeriensis | 49.4 | 24.6 | 1.58 | 22.6 | Sánchez-Zurano et al. (2021) |
| Haematococcus pluvialis | 32.59 | 0.13 | 3.24 | 34.56 | Marinho et al. (2021) |
| C. vulgaris | 45.64 | 59.71 | 19 | 21.16 | Ricky et al. (2022) |
Bioactive Constituents and Pharmaceutical Properties of Microalgae
Microalgae are acknowledged as a prolific and underutilized source of bioactive chemicals with significant potential for many uses. These microscopic photosynthetic organisms may manufacture a variety of primary and secondary metabolites, many of which possess valuable nutritional, medicinal, and industrial characteristics (Alghazeer et al., 2022). Typically, microalgae have been cultivated mainly for the synthesis of several recognized beneficial chemicals, including carotenoids, critical omega-3 fatty acids, and phycobilipigments (Quintas-Nunes et al., 2023). Recent study has revealed the significant untapped potential of microalgae, emphasizing the existence of several bioactive compounds with prospective nutraceutical and medicinal applications (Alghazeer et al., 2022; Mobin and Alam, 2017).
Microalgae have attracted interest for their capacity to generate a wide variety of bioactive secondary metabolites. These encompass substances possessing antioxidant, anti-inflammatory, antibacterial, and potential anti-cancer activities (Alghazeer et al., 2022; Mobin and Alam, 2017; Saha and Murray, 2018). The chemical composition and quantity of these bioactive molecules can fluctuate considerably based on factors such as species, growth circumstances, and environmental influences (Alghazeer et al., 2022; Quintas-Nunes et al., 2023). Bioactive molecules are typically secondary metabolites, encompassing a diverse array of chemicals such as organic acids, carbohydrates, amino acids, peptides, vitamins, growth regulators, antibiotics, enzymes, and poisonous compounds (de Morais et al., 2015; Munaro et al., 2021). The metabolites exhibit a diverse array of biological actions, encompassing anticancer, antiviral, antioxidant, and immunomodulatory properties (Arain et al., 2022a; Du et al., 2024). The possibility exists for the discovery of novel medication leads from these metabolites. Cyanobacteria are the most significant source of bioactive chemicals among numerous groups of microalgae (Martínez-Francés and Escudero-Oñate, 2018). In summary, the extensive diversity and unexploited potential of bioactive chemicals in microalgae signify a promising frontier in nutrition, medicines, and sustainable resource management.
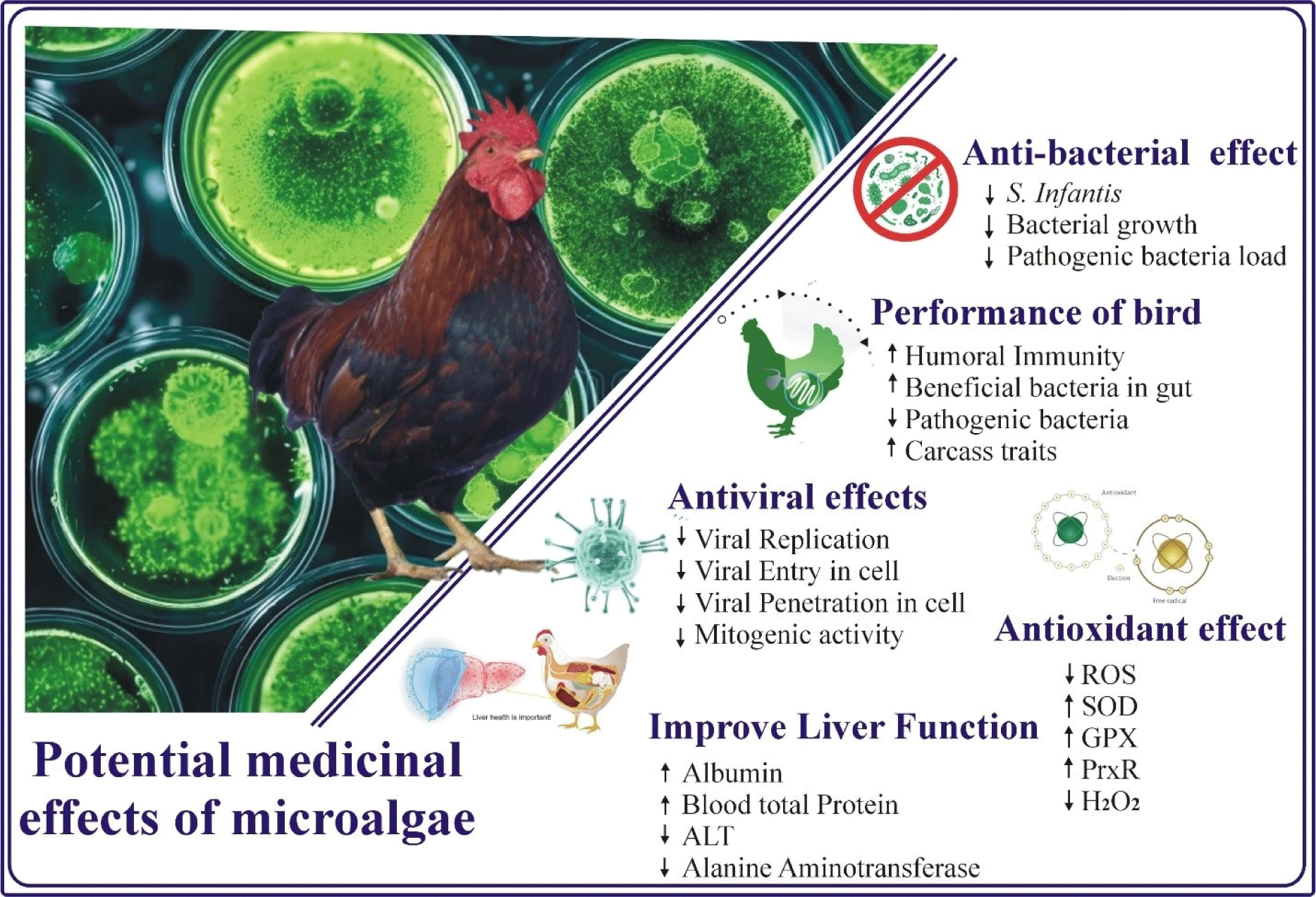
Microalgae possess numerous pharmaceutical and nutraceutical properties, offering potential benefits for poultry health and performance. Research indicated that microalgae contains bioactive compounds such as carotenoids, omega-3 fatty acids, antioxidants, vitamins, and minerals, they are participated to enhance the immune responses and reduce oxidative stress in poultry (Abdelnour et al., 2019; Fig. 1). These bioactive compounds enhance the blood concentration of anti-oxidant enzyme like superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT), thereby reduced the oxidative stress by improving the antioxidant defense (Emam et al., 2024; Panaite et al., 2023). Furthermore, dietary supplementation of microalgae significantly reduces the oxidative stress by scavenging toxic radicals of reactive oxygen species (ROS) and also mitigated the negative effects of heat stress in broiler chicken (Chaudhary et al., 2023a). Another study, have revealed that mesobiliverdin IXα (MBV)-enriched microalgae extracts can improve gut health and microbial balance in broilers, increasing beneficial Lactobacillus species and promoting intestinal villi growth. Notably, MBV-SP2 lowered pro-inflammatory markers and improved intestinal morphology more effectively than antibiotic treatments, highlighting MBV-enriched extracts as a promising alternative for antibiotic-free broiler health management (Chang et al., 2021). Previously it was demonstrated broiler fed diet containing a mixture of Dunaliella salina and Spirulina (1.0 and 1.5 g/kg), significantly enhanced oxidative stress, body weight, feed efficiency, and immune response, in addition to boost the lipid profiles, liver and kidney function (Alghamdi et al., 2024). Additionally, microalgae possess antimicrobial and anti-inflammatory properties, helping to combat pathogens and reduce disease incidence (Pagels et al., 2022). As a natural and sustainable feed additive, microalgae present a promising strategy for boosting poultry health and productivity without relying on synthetic drugs (Fig. 1). Previously (Balakrishnan et al., 2021), suggested that fatty acid methyl esters derived from marine microalgae effectively inhibit Listeria monocytogenes growth, demonstrating potent antimicrobial properties by disrupting cellular metabolism and membrane integrity. Results also confirmed that fatty acid esters suppressed the biofilm formation and improved Caenorhabditis elegans survival in infection models, showing potential as a natural feed additive for poultry to control infection and Listeria contamination (Balakrishnan et al., 2021).
Prooxidants are typically xenobiotic or external compounds, such as certain chemicals, pollutants, and environmental toxins, they are responsible to promote the oxidative stress by increasing the production of free radicals of ROS and disrupted the body ability to neutralized these toxic agent (Rahal et al., 2014). Reactive molecules of oxygen derivatives produced during the process of ROS formation like superoxide anion (O2–), hydrogen peroxide (H2O2) and hydrogen radicles (OH; Bergamini et al., 2004). The excessive production of these radicals interrupt the normal physiological function at cellular level, leading to cause serious damage and initiated the development of numerous pathological conditions including cancer, cardiovascular and neurodegenerative disorders (Arain et al., 2018).
Oxidative stress in poultry refers to the imbalance between the production of ROS and the bird’s ability to counteract these harmful molecules with antioxidants. Poultry birds are more susceptible to oxidative stress specially under intensive farming and heat stress conditions particularly in tropical and subtropical regions of the world. Several strategies have been implemented to reduce the oxidative stress such as adopting modern technologies, improved management, shifting of conventional forming practices into the environmental control farming practices and dietary manipulation (Saeed et al., 2017a). Several studies have been reported that nutritional intervention and use phytogenic feed additive (herbs and their derivative) are the effective strategy to reduce the incidence of heat stress and mitigate the negative effects of oxidative damage (Arain et al., 2024; Changxing et al., 2018; Hassan et al., 2023; Saeed et al., 2017b). Furthermore, it was demonstrated that antioxidants supplied through external dietary supplements, they play a crucial role in combating oxidative stress by scavenging ROS and boosting the activity of antioxidant enzyme in poultry birds (Aboamer et al., 2024; Ashraf et al., 2024).
Microalgae have recently attracted considerable interest for their exceptional potential as a source of vital bioactive chemicals, especially antioxidants. The bioactive substances found in microalgae, such as bioflavonoids, carotenoids, vitamins C and E, biometals, and omega-3 and 6 fatty acids, are particularly effective to regulate the oxidative stress and boost the anti-oxidant defense (Fernandes et al., 2020b; Kiran and Venkata Mohan, 2021). Microalgae naturally contains numerous antioxidant enzymes including SOD, CAT, GPx, and peroxiredoxin which play a crucial role in neutralizing the free radicals of ROS. These antioxidants either directly scavenge harmful radicals or regenerate other antioxidants, utilizing the reducing power generated through photosynthesis (Cirulis et al., 2013).
Multiple studies have documented the existence of a wide range of antioxidant chemicals in several microalgal species, such as Chlorella, Spirulina, and Dunaliella, among others (Coulombier et al., 2021). The antioxidant capabilities of microalgae stem from their capacity to neutralize free radicals, impede lipid peroxidation, and safeguard cells from damage generated by oxidative stress (Lucas et al., 2020; Saha and Murray, 2018; Sikiru et al., 2019).
Previous research has examined the antioxidant capabilities of Chlorella vulgaris, a green microalga recognized for its substantial nutritional benefits and medicinal attributes. C. vulgaris exhibits significant antioxidant activity, exceeding that of widely utilized synthetic antioxidants (Sikiru et al., 2019). The antioxidant characteristics of C. vulgaris are mainly attributed to the presence of abundant amount of carotenoids, vitamins, and several bioactive substances (Hamouda et al., 2022). Similarly, Spirulina, a blue-green microalga, has been thoroughly studied for its antioxidant characteristics, and recognized as a substantial source of phycocyanin, a powerful antioxidant pigment that demonstrates several therapeutic effects, including anti-inflammatory, neuroprotective, and anticancer properties (Alghazeer et al., 2022; Mamani et al., 2020; Sikiru et al., 2019). Similarly, Caulerpa filiformis, a green species of algae found in the Sechura Bay area of Peru, renowned for its powerful antioxidant potential, owing to the presence of numerous bioactive compounds (Laos et al., 2023). Besides their antioxidant characteristics, microalgae have been explored for possible applications in biofuel generation, wastewater treatment, and animal feed augmentation. The antioxidant capacity of microalgae is well-documented, and their utilization as a source of natural antioxidants presents a promising research avenue with considerable implications for enhancing the health and productivity of livestock animals (Mobin and Alam, 2017).
Inflammation is a key physiological process essential for the body’s defense against detrimental stimuli, like pathogens, damaged cells, or irritants (Rock et al., 2009). This intricate reaction encompasses a series of cellular and molecular activities aimed at eradicating the primary source of cellular harm, removing necrotic cells and tissues affected by the initial damage, and commencing the healing process.
The anti-inflammatory characteristics of microalgae derived from their diverse secondary metabolites, have demonstrated the ability to regulate immune system and reduced inflammatory reactions (Tolba et al., 2020). Microalgal species such as C. filiformis exhibit significant antioxidant and anti-inflammatory properties, perhaps attributable to their elevated levels of polyphenolic chemical (Pagels et al., 2022). Glycoglycerolipids, a category of glycolipids sourced from microalgae, have emerged as potential anti-inflammatory medicines. These compounds have been shown to possess several biological actions, including anti-inflammatory, antiviral, anticancer, and antibacterial properties, rendering them promising candidates for therapeutic development (Conde et al., 2021). Furthermore, microalgae-derived metabolites, including polyunsaturated fatty acids, carotenoids, and polysaccharides, play a pivotal role in suppressing the synthesis of pro-inflammatory mediators (Choo et al., 2020). These bioactive compounds inhibit the production of cytokines such as TNF-α, IL-1β, and IL-6, which are critical drivers of inflammation (Ávila-Román et al., 2021). Additionally, microalgae metabolites modulate the arachidonic acid pathway, reducing eicosanoid synthesis, including prostaglandins and leukotrienes (Harwood, 2023). This anti-inflammatory action occurs through the downregulation of NF-κB and COX-2 signaling pathways (Nabil-Adam et al., 2023; Talero et al., 2015). These metabolites offer potential therapeutic applications in managing chronic inflammatory conditions and promoting overall health by mitigating the inflammatory cascade effectively. In summary, the anti-inflammatory potential of microalgae represents a promising research domain, with several studies emphasizing the variety of bioactive chemicals generated by these microscopic entities.
Recent research indicates that microalgae contain distinctive bioactive chemicals that demonstrate significant antibacterial activity, presenting a viable alternative to conventional antimicrobial treatments (Ferrazzano et al., 2020). A multitude of studies has investigated the antibacterial properties of various microalgal species. Cyanobacteria and microalgae are recognized as prolific sources of diverse bioactive compounds, such as polyphenols, terpenoids, and carotenoids, which exhibit antimicrobial properties against several pathogenic bacteria (Ferrazzano et al., 2020; Mamani et al., 2020). Furthermore, Chlorella, Spirulina, and Dunaliella produce secondary metabolites, including peptides, polysaccharides, phenolics, and fatty acids, that exhibit potent antimicrobial properties (Ilieva et al., 2024; Rojas et al., 2020). The primary mechanism of action behind the antimicrobial effects of these compounds is to disrupt the integrity of microbial cell membranes (Stirk and van Staden, 2022). Additionally, fatty acids like polyunsaturated lipids and certain peptides in microalgae destabilize bacterial and fungal membranes, causing leakage of cellular contents and eventual cause cell death (Surendhiran et al., 2021). Moreover, microalgae-derived phenolics and flavonoids inhibited the activity of microbial enzymes disturb the metabolic pathways crucial to maintain the normal growth and multiplication of microbial cells (Hassan et al., 2022b). Another mechanism involved to inhibit the bacterial growth, microalgae produce ROS and antioxidant compounds (carotenoids and phycocyanin), that create oxidative stress within microbial cells, impairing DNA, proteins, and lipid integrity leading to cause cell death (Hamidi et al., 2019). In addition to the direct anti-microbial effects, microalgae also modulate gut microbiota by promoting beneficial microbial growth while suppressing pathogenic species (Ma et al., 2022). This competitive inhibition is attributed to the production of antimicrobial metabolites and improve the immunological status and gut barrier functions, thereby improving the health and proactive performance of livestock animals.
The antibacterial capabilities of microalgae can be affected by several factors, including growing conditions, extraction procedures, and specie of organism. A study on Spirulina platensis shown that the supercritical fluid extracts of this microalga displayed significant antibacterial activity against gram-positive and gram-negative bacteria, as well as yeasts and fungi (Mendiola et al., 2007). Similarly another study, demonstrated that microalgal species such as Chlorella, Dunaliella, and Nostoc produce bioactive chemicals having with superior antibacterial characteristics (Saha and Murray, 2018). The antibacterial capabilities of microalgae signify a viable direction for additional research and development by utilizing varied bioactive molecules generated by these photosynthetic organisms.
Advantageous Utilization of Microalgae in Poultry
Microalgae possess considerable potential to enhance growth performance and general health in poultry (Table 2). These microorganisms exhibit an extraordinary capacity to flourish under diverse environmental settings, rendering them a versatile and sustainable feedstock alternative. Multiple studies have evidenced the beneficial effects of integrating microalgae into poultry feed on several growth metrics, including body weight, feed conversion ratio, and egg production (Benedetti et al., 2018; de Morais et al., 2016).
| Type of animal | Type, dosage and duration | Mechanism | Overall effect | References |
|---|---|---|---|---|
| Broilers and layers | Spirulina (1.5%, 3.0%, 6.0%, or 12.0% for nine weeks | ↑ Cellular and humoral immunity, ↑ Maintaining the beneficial intestinal bacteria ↓ Pathogenic bacteria ↑ Improving the carcass traits |
Improves productive performance of broilers and layers | El-Ghany (2020) |
| Broilers | 1%–2% micro algae in diet for 42 days | ↑ Superoxide dismutase (SOD) ↓ Polyunsaturated fatty acid ↓ Malondialdehyd |
Improves performance, serum composition, carcass trait, antioxidant status, and fatty acid | El-Bahr et al. (2020) |
| Broilers | Microalgae Chlorella spp. 0.5%–1.0% of the diet | ↑ Blood total protein, albumin ↑ High-density lipoprotein (HDL) cholesterol ↓ Alanine aminotransferase and ALT ↑ Blood lymphocytes ↑ IgA, IgG, and IgM |
↑ Body weight gain ↓ Feed conversion ratio ↓ Drip loss ↑ Liver function ↑ Immunity |
Abdelnour et al. (2019) |
| Cornish cross chicks | Docosahexaenoic acid (DHA; 22:6n-3)-rich 2% microalgae for day 42 | ↑ Breast muscle ↑ Meat tenderness and color ↓ Incidence of breast muscle striping and myopathy |
Improves production performance, breast muscle quality attributes, lipid profile, and incidence of white striping and myopathy | Khan et al. (2021) |
| Isa brown laying hens | DHA-rich microalga 0.25%, 0.5%, and 1.0% for 40 d | ↑ Nutrititive value of hen’s eggs | ↑ Omega-3 content of eggs | Moran et al (2018); Moran et al. (2019) |
| Broiler chickens | Microalgae Tetraselmis chuii 20 g/kg of feed (2%) | ↓ S. Infantis caecal load | Antibacterial | Corrales-Martinez et al. (2022) |
| Broiler chickens | Microalgal DHA 2% for 6 weeks | ↑ Body weight gain ↓ Cholesterol and triglyceride concentrations in plasma, liver, breast, and thigh ↑ Improve tibia breaking strength ↑ Total bone volume and bone mineral |
Improves growth performance, tissue lipid profiles, and tibia characteristics | Kalia et al. (2023) |
| Fayoumi broilers | Microalgae (Spirulina platensis) 1% for 8 weeks | ↑ Serum total protein ↑ Globulin ↓ Serum cholesterol ↑ Lymphocyte percentage |
Improves growth performance, ingestive behavior, hemato-biochemical parameters, and economic efficiency | Hassan et al. (2022a) |
| Broiler and layer birds | Microalgae | ↑ Weight, Feed intake and digestibility ↑ Increase meat quality ↑ Improve organoleptic quality of meat ↑ Digestibility ↑ Egg shell thickness and egg weight |
Enhances the nutritive value of poultry meat an eggs | Esakkimuthu et al. (2024) |
| Broiler and layer birds | S. platensis (0.25%–1.0%) and Chlorella vulgaris (1.55 g/kg) | ↓ Oxidative stress ↑ Immune response, growth rates, feed conversion ratios ↑ Carcass quality, and meat attributes ↑ Egg production and egg quality |
Positive impact on performance metrics | Abdel-Wareth et al. (2024) |
| Broiler | S. platensis (10%) and Haematococcus pluvialis (0.004%) | ↑ GSH, glutathione peroxidase (GPx) and SOD | Improves antioxidant status | Abdel-Wareth et al. (2024); Jubie et al. (2012) |
| Broilers | C. vulgaris spp.; CLV; 0.5%–1.0% of the diet) | ↑ Body weight gain (2.7%) ↑ Feed conversion ratio (lowered by 2.8%) ↑ Meat color and breast muscle weight (20.1%) ↓ Drip loss (2.26%) from breast muscle ↑ IgA (29.7%,), IgG (69.1%), and IgM (32.3%) |
Improves growth and health of birds | Abdelnour et al. (2019) |
| Ross-308 broiler chicks | 1 g/kg diet of C. vulgaris (CV), S. platensis, and Amphora coffeaformis (AC) | ↑ Essential fatty and amino acids ↓ Microbial growth in breast muscle ↓ Malondialdehyde (MDA) and protein carbonyl (PC) levels, cooking loss and aerobic plate count (APC) ↑ SOD activities in breast muscle |
Enhance performance and meat quality in broiler chickens | El-Bahr et al. (2020) |
Three kinds of microalgae, namely C. vulgaris, S. platensis, and Amphora coffeaformis, were reported to positively influence body weight, growth performance, and meat quality in broiler chickens (El-Bahr et al., 2020). The results indicated that microalgae substantially enhanced the profiles of important fatty and amino acids, increased antioxidant levels in breast muscles, and diminished microbial development and oxidative damage (El-Bahr et al., 2020). Another study indicated that supplementing broiler diets with microalgae, with or without xylanase, markedly enhanced growth performance, increasing body weight by over 44% and decreasing feed conversion ratio by 6.3%. They further suggested possible advantages for gut health, evidenced by notable alterations in gut health genes and an expanded villi surface area in the microalgae groups (Chaudhary et al., 2023a). Another study examined the effects of S. platensis supplementation in Fayoumi hens, demonstrating that a diet containing 1% S. platensis markedly enhanced growth performance and immunological response. Economically, 0.25% S. platensis was determined to be the most cost-effective, optimizing production and profitability (Hassan et al., 2022a). Moreover, the pilot investigation indicated that food supplementation with Tetraselmis chuii and Porphyridium cruentum microalgae enhanced body weight and gut morphology in broiler chicks. Treated birds exhibited increased villus-height-to-crypt-depth ratios, less thawing weight loss in fillets, and higher nutrient absorption, indicating a viable approach for augmenting growth performance (Šefcová et al., 2021). Supplementation of heat-stressed broilers with 3% microalgae markedly increased final body weight and improved gut health indicators, encompassing antioxidant, immunological, and tight-junction gene expression. Furthermore, microalgae enhanced microbial diversity and fostered good gut bacteria, illustrating its potential to alleviate heat stress in chickens (Chaudhary et al., 2023b). Enhancing the diets of Arbor Acres chicks with DHA-enriched microalgae markedly enhanced growth performance, liver proportion, and antioxidant status, while decreasing abdominal fat and blood cholesterol levels. Furthermore, birds that consumed microalgae exhibited enhanced deposition of good fatty acids and decreased oxidative stress relative to the control group (Long et al., 2018). The published research demonstrates that microalgae is a significant and dependable feed element for enhancing the productive performance of chickens (Fig. 2).
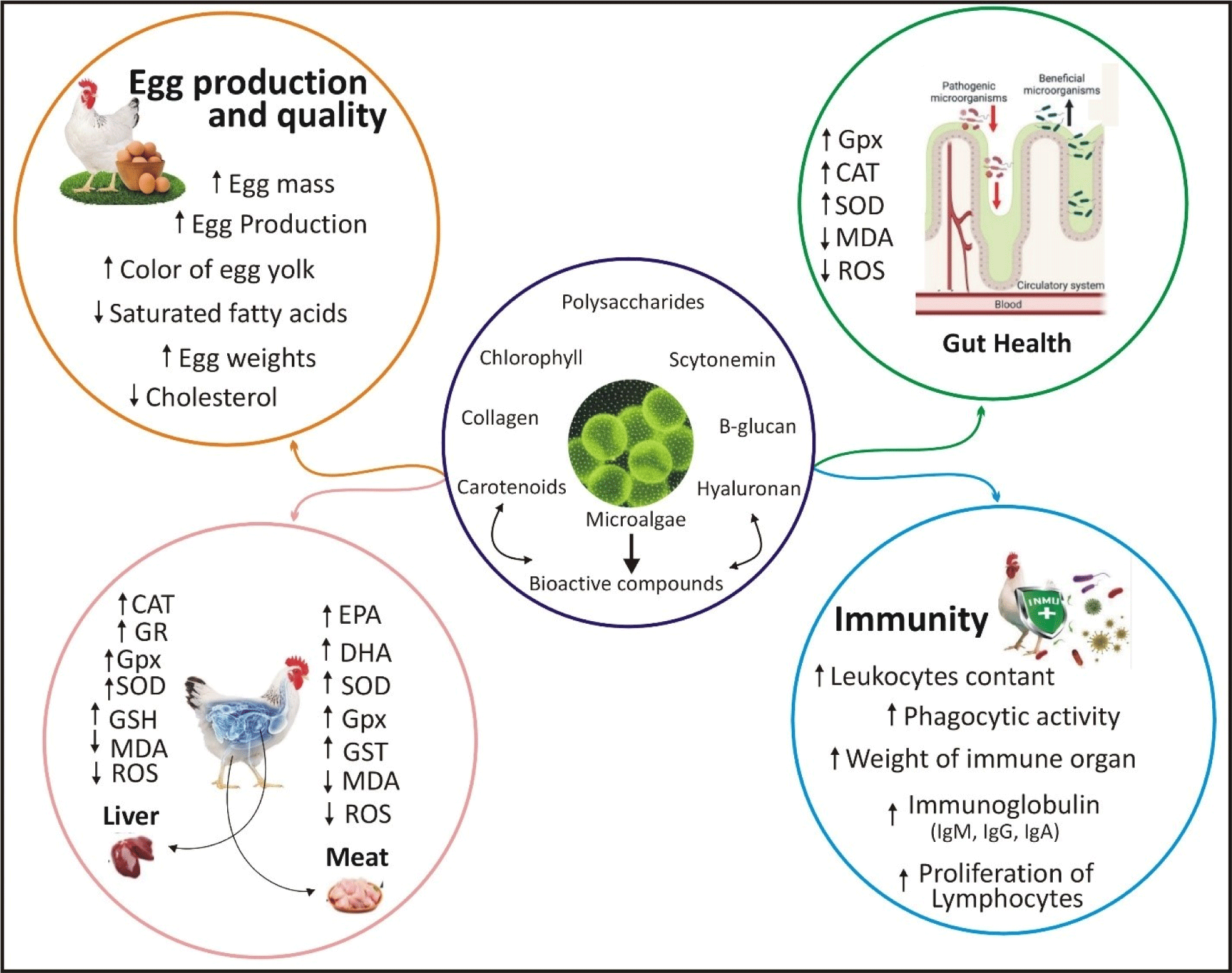
Integrating microalgae into poultry feed offers the significant benefit of potentially enhancing the nutritional quality of the meat (Martins et al., 2021). In addition, microalgae containing carotenoids and other bioactive compounds that can enhance the color and overall quality of poultry meat. Studies have shown that adding ω-3-rich microalgae (Schizochytrium JB5) to broiler diets improved the fatty acid composition of breast meat by increasing levels of DHA, oleic acid, and ω-3 fatty acids, while decreasing the ratio of ω-6 to ω-3 and the saturated fat content (Gatrell et al., 2015). Despite these advantages, the growth performance and blood parameters of the poultry were not impacted by the inclusion of microalgae in their diet (Yan and Kim, 2013). Furthermore, a research examined the impact of various microalgae species on broiler chickens. It demonstrated that S. platensis notably enhanced the crucial fatty and amino acid compositions while decreasing microbial growth in the breast muscle (Amer et al., 2024). In addition, all microalgae types decreased the markers of oxidative stress and enhanced meat quality by reducing melondialdehyde and protein carbonyl levels, as well as cooking loss (Varzaru et al., 2024). These results indicated that microalgae have the potential to enhance broiler performance and meat quality. On the contrary, a recent study on feeding trials examined the impact of incorporating microalgal biomass into broiler diets. The results revealed that substituting 5%–10% of soybean meal with three different microalgal strains led to a reduction in breast weight percentages, while not impacting the overall growth performance or meat quality (Cabrol et al., 2022). It is worth noting that the combined use of microalgae did not prevent the loss of breast weight and also influenced gene expressions associated with muscle hypertrophy and atrophy, indicating complex effects on protein metabolism (Sun et al., 2024). In addition, Liu and his collogues studied that laying hens fed diet supplemented with DHA-rich microalgae (Aurantiochytrium sp.) led to a significant enhancement in the n-3 polyunsaturated fatty acid levels and lipid health indicators of breast and thigh meat (Liu et al., 2022). The enrichment did not affect meat quality aspects such as tenderness or color, however slight increase in lipid oxidation was observed in cooked and stored meat (Liu et al., 2022). Furthermore, incorporating Hermetia illucens larval meal or spirulina into the poultry diet, showed slightly increase in color and saturated fat content (Gkarane et al., 2020). Similarly, another study reported that spirulina supplementation significantly improved the red color of chicken and enhance the umami flavor of meat after storage under oxygen-rich environment (Altmann et al., 2020). Furthermore, dietary incorporation of alternative protein sources such as Spirulina and Hermetia meal to poultry feed demonstrates encouraging outcomes for the quality of meat, especially when combined with advanced packaging techniques (Altmann, 2020). Moreover, broiler chicken diets enriched with DHA-rich microalgae and methionine led to a significant decrease in the occurrence of breast muscle white striping and myopathy, along with an improvement in the muscle’s n-3 fatty acid content (Khan et al., 2021). Nevertheless, the incorporation of methionine resulted in increased meat tenderness and changes in its color, suggesting possible trade-offs in meat quality (Khan et al., 2021). Conclusively, incorporating microalgae into poultry feeds has revealed a substantial potential and great promise in improving efficiency and supporting the creation of premium poultry goods.
Exploring the use of microalgae in poultry feed has been considered to enhance egg production, as these tiny organisms can provide important nutrients like proteins, lipids, and pigments (Fernandes et al., 2020a). C. vulgaris and S. platensis are an examples of microalgae that contain various bioactive compounds capable of positively impacting the health and performance of laying hens. Incorporating microalgae into poultry diets not only boosts egg production but also enhances the nutritional content and sensory characteristics of the eggs (Liu et al., 2020). The presence of abundant amount of carotenoids and fatty acids in microalgae biomass, significantly improve the eggs’ color, texture, and overall quality, making them more attractive to consumers (Kalia and Lei, 2022).
Research has shown that integrating microalgae into chicken feed can cause a notable rise in egg output. The active components found in microalgae, like carotenoids and polyunsaturated fatty acids, can boost the metabolic functions of the birds, improving nutrient absorption and energy effectiveness (Vlaicu et al., 2023). Additionally, superior nutritional value of microalgae helps to boost growth performance and optimize the development of reproductive system of laying hens leading to increased egg production (Martins et al., 2021). Preceding studies have shown that the active components in microalgae, like carotenoids and polyunsaturated fatty acids, can boost the metabolic functions of the birds, improving nutrient absorption and energy effectiveness (Emam et al., 2024). In addition, the supplementation of S. platensis to the feed for layers resulted in noticeable enhancements in egg production, egg quality, and indicators of blood health (Abbas et al., 2022). The most effective level of S. platensis inclusion was found to be 9%, with 4.7% of soybean meal being replaced for every 1% of S. platensis, indicating its viability as a cost-effective dietary alternative (Abbas et al., 2022).
During a 32-week experiment, the addition of graded levels of All-G-RichTM microalgae in layer diets resulted in a notable elevation of DHA levels in egg yolks, with no impact on production performance, egg weight, or shell quality (Ao et al., 2015; Keegan et al., 2019). Simultaneously, dietary supplementation of microalgae oil or fish oil to the diet of laying hens raised DHA levels and n-3 PUFA in eggs, encouragingly both sources were found equally effective (Kaur et al., 2024). Nevertheless, eggs produced from microalgae oil had a better taste and were more generally accepted, indicating that it could be a more favorable choice for enriching DHA compared to fish oil (Feng et al., 2020). Likewise, the addition of omega-3-rich microalgae such as Phaeodactylum tricornutum, Isochrysis galbana, and Chlorella fusca to the diets of laying hens resulted in a significant increase in n-3 long-chain polyunsaturated fatty acids (LC-PUFA) in egg yolk, particularly DHA (Lemahieu et al., 2013). It is important to note that C. fusca mainly boosted α-linolenic acid, while Phaeodactylum and Isochrysis demonstrated the highest LC-PUFA efficiency, also improving yolk color through carotenoid transfer (Lemahieu et al., 2013). Whereas another study demonstrated that enhancing the diets of Japanese quail with Spirulina and Dunaliella (SD) resulted in better body weight, feed conversion ratio, and fertility, along with remarkable decreases in lipid profile indicators (Abd El-Hack et al., 2024). Despite minimal changes in egg production and quality, the use of SD improved the function of the liver and kidneys, as well as the quails’ immunological response (Abd El-Hack et al., 2024). Convincingly, it was noted that adding microalgae to the diet not only enhances egg production but also elevates the nutritional quality of poultry eggs.
Potential Drawbacks of Adding Microalgae to Poultry Feed
The widespread use of microalgae in poultry diets has been impeded by various constraints that require significant attention. A key obstacle is the inconsistent quality and makeup of microalgae, which can be impacted by several factors like cultivation conditions, harvesting methods, and processing techniques (Martins et al., 2021). Henceforth, formulating poultry diets that consistently meet the birds’ specific nutritional requirements can be challenging due to the variability in ingredients. Additionally, the high expense of producing and processing microalgae may render it economically impractical for large-scale commercial poultry operations, particularly when compared to traditional protein sources like soybean meal and fishmeal (Ciani et al., 2021; Sanjari et al., 2018).
Microalgae’s potential to cause unwanted sensory characteristics in poultry products, such as off-flavors or texture alterations, presents another limitation (Espinosa-Ramírez et al., 2023). This limitation can significantly hinder consumer acceptance, especially for eggs and meat, which are highly sensitive to flavor and quality. Furthermore, incorporating microalgae into poultry diets can be difficult due to the requirement for specialized feed processing equipment and the possibility of decreased feed intake or digestibility in the birds. Despite these constraints, continuous research and technological progress could potentially surmount the obstacles to the extensive utilization of microalgae in poultry nutrition. Innovative methods of cultivation, such as utilizing wastewater or industrial waste streams as nutrient sources, may aid in cutting production expenses and enhancing the sustainability of feeds based on microalgae.
Conclusion
Microalgae holds significant potential as a nutraceutical and alternative protein source for the poultry industry, offering a sustainable, nutrient-rich solution to enhance poultry health and productivity. It is widely accepted that microalgae are a valuable source of essential nutrients, such as proteins, omega-3 fatty acids, vitamins, and antioxidants, which help to boost immune status, enhance nutrient utilization, reduce oxidative stress and prevent infectious disorders, thereby improves health status and overall productive performance. Furthermore, microalgae contribute to gut health by modulating gut microbiota and improving intestinal integrity, thus decreasing the incidence of diseases. Additionally, inclusion of microalgae in poultry diets significantly enhance quality of meat and egg by improving the nutrient content and reducing undesirable fatty acids, these benefits make them a valuable feed additive for modern poultry to ensure the consistent productivity.













