Introduction
Elucidating the relationship between probiotics and the deconjugation of bile salts through the activity of bile salt hydrolases (BSH; EC.3.5.1.24) remains an interesting topic. Duary et al. (2012) and Zhang et al. (2009) reported that Lactobacillus constitutively expressed BSH, which was slightly upregulated in the presence of bile acid. Furthermore, Begley et al. (2006) highlighted that most BSH activity is detected in gram-positive bacteria, specifically probiotic candidates. However, variation in enzyme activity across strains was significant, and the exact function or mechanism of the enzyme remains unclear (Horackova et al., 2020; Urdaneta and Casadesús, 2017; Yang et al., 2019).
Clarifying the exact mechanism of BSH activity is important. As reports of probiotics reveal blood cholesterol level reduction, potentially enabling further development of therapeutic applications (Agolino et al., 2024; Ahn et al., 2003; Begley et al., 2006; Mann and Spoerry, 1974). For modern people, Feingold (2016) reported elevated blood low-density lipoprotein (LDL)-cholesterol levels were observed, a high-risk factor for cardiovascular disease (CVD), possibly resulting from modern diets and high-nutrient ingredients. As a result, probiotics are emerging as important health components of food for the public and patients with high cholesterol levels. Drugs that manage blood LDL-C levels include statins and their derivatives, which lower cholesterol synthesis in the liver (Feingold, 2016), and ezetimibe, which prevents the reabsorption of bile acids, promoting excretion from the body (Florentin et al., 2008; Kashani et al., 2008). However, the side effects of these medications are concerning (i.e., muscle complications, myopathy, acute pancreatitis, liver toxicity, and increased risk of diabetes; Florentin et al., 2008; Kashani et al., 2008), which need the development of new compounds with fewer side effects. Accordingly, developing and commercializing BSH, a high-possibility reason for lower blood cholesterol (Agolino et al., 2024; Begley et al., 2006; Guo et al., 2012), as a drug or supplement (e.g., postbiotics or genetically extracted by cloned vector) is expected.
However, BSH, a candidate for cholesterol control, remains uncommercialized because the potential risks have not been identified. For example, Sun et al. (2023) analyzed the intestinal contents of patients with colorectal cancer (CRC) and found that secondary bile acid levels increased significantly by BSH activity (Evangelakos et al., 2021; Perez and Briz, 2009; Sun et al., 2023). Secondary bile acids can cause inflammatory responses, cell membrane destruction, and DNA damage. This affects the intestinal cells, ultimately leading to CRC (Ajouz et al., 2014). Another potential risk is BSH may act as an antibiotic resistance factor. Kusada et al. (2022a) reported that Lactobacillus paragasseri JCM 5343T has antimicrobial resistance (AMR) by BSH activity, which can pose an antibiotic resistance when transformed into other bacteria (Daly et al., 2021). Because potential risks of BSH are high, it is currently difficult to use for clinical purposes.
It is still difficult to understand why probiotics synthesize BSH and deconjugate bile salt. To get enough data, it is more important to understand the exact mechanism of BSH. Furthermore, it is impossible to stop the synthesis of BSH by probiotics or gut microbiome due to various safety and ethical issues regarding genetic manipulation. Therefore, this review pointed to how differences appear by species or phylogenetic tree through the substrate specificity analysis and makes foundation for further research.
In this review, 122 published articles on BSH and probiotics were examined. These studies explained BSH activity according to taxa in the past three decades. They were sourced from electronic databases, including Public/Publisher MEDLINE (PubMed), Google Scholar, National Center for Biotechnology Information (NCBI), American Type Culture Collection (ATCC), American Society for Microbiology (ASM) journals, SpringerLink, Food Research International, Multidisciplinary Digital Publishing Institute (MDPI), Journal of Dairy Science, Frontiers, Korea Science, Proceedings of the National Academy of Sciences (PNAS), Animal Bioscience (AB), Royal Society, Nature, Institute of Food Technologists (IFT), Wiley-online library, British Medical Journal (BMJ), Europe PMC, Science Direct, Research Gate, Talyor and Francis Online, Cambridge University Press, Journal of Lipid Research (JLR), Public Library of Science (PLOS), Tennessee University Libraries, AUMA publication, Atherosclerosis Journal and OXFORD Academic. The keywords used were bile salt, bile acid, BSH, penicillin V acylase (PVA), blood cholesterol-lowering effect, probiotics, hydrogel formation, and antibiotic growth promoters (AGPs). Main review address information regarding BSH mechanisms and activities across lactic acid bacteria (LAB) species are discussed. Additionally, the structures and functions of BSH and PVA are compared. Finally, the current challenges and possible solutions, focusing on the potential use of BSH in clinical settings, are highlighted.
Function and Activity of Bile Salt Hydrolase
Bile salt and bile acid are distinguished if glycine or taurine is conjugated or not. If no glycine or taurine is attached, the substance is referred to as bile acid; otherwise, it is called bile salt (Daly et al., 2021). Primary bile acids are synthesized in the liver and denatured by bacteria to form secondary bile acids (Daly et al., 2021). Haslewood (1967) reported that the primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), are found in the bile of vertebrates. Similarly, secondary bile acids, including deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid, are found in this fluid (Haslewood, 1967). Notably, DCA is produced by modifying CA, and LCA is derived from CDCA (Bachmann et al., 2015; Evangelakos et al., 2021; García-Cañaveras et al., 2012; Perez and Briz, 2009). Tagliacozzi et al. (2003) and Thakare et al. (2018) reported that CDCA predominated in human plasma, followed by deoxycholic acid (DCA), and glycochenodeoxycholic acid was three times higher than taurochenodeoxycholic acid.
A wide range of bile acids are distributed across different species (Kuhajda et al., 2006; Li and Dawson, 2019). Bile salts conjugated with taurine are dominant in most cases, excluding those in humans and other animals. In addition, Karakus et al. (2024) reported that glycine-conjugated bile salts are dominant in humans, and taurine-conjugated bile salts are most common in dogs (García-Cañaveras et al., 2012; Kakimoto et al., 2017; Rabin et al., 1976; Vessey, 1978).
Bile salts primarily aid in food digestion (Maldonado-Valderrama et al., 2011). de Buy Wenniger and Beuers (2010) and Redinger (2003) reported that bile salts are synthesized in the liver, stored in the gallbladder, and secreted along with pancreatic enzymes in the duodenum upon ingestion of food. Notably, these salts have amphipathic characteristics (Daly et al., 2021). The hydrophobic part attaches to ingested lipid droplets and divides them into smaller particles. These fine lipid particles help lipolytic enzymes, such as lipase, to work better, and the bile salt is reabsorbed 95% near the ileum when the process is complete. It enters the portal vein along the capillaries and re-enters the liver thereafter. The 5% of bile salt that was not absorbed from the ileum was fermented or deconjugated by the gut microbiome. Most of the affected bile salt is excreted with feces but some of it is reabsorbed. The whole circulation and enzyme effect for bile circulation is shown in Fig. 1 (Daly et al., 2021; de Buy Wenniger and Beuers, 2010; Redinger, 2003).
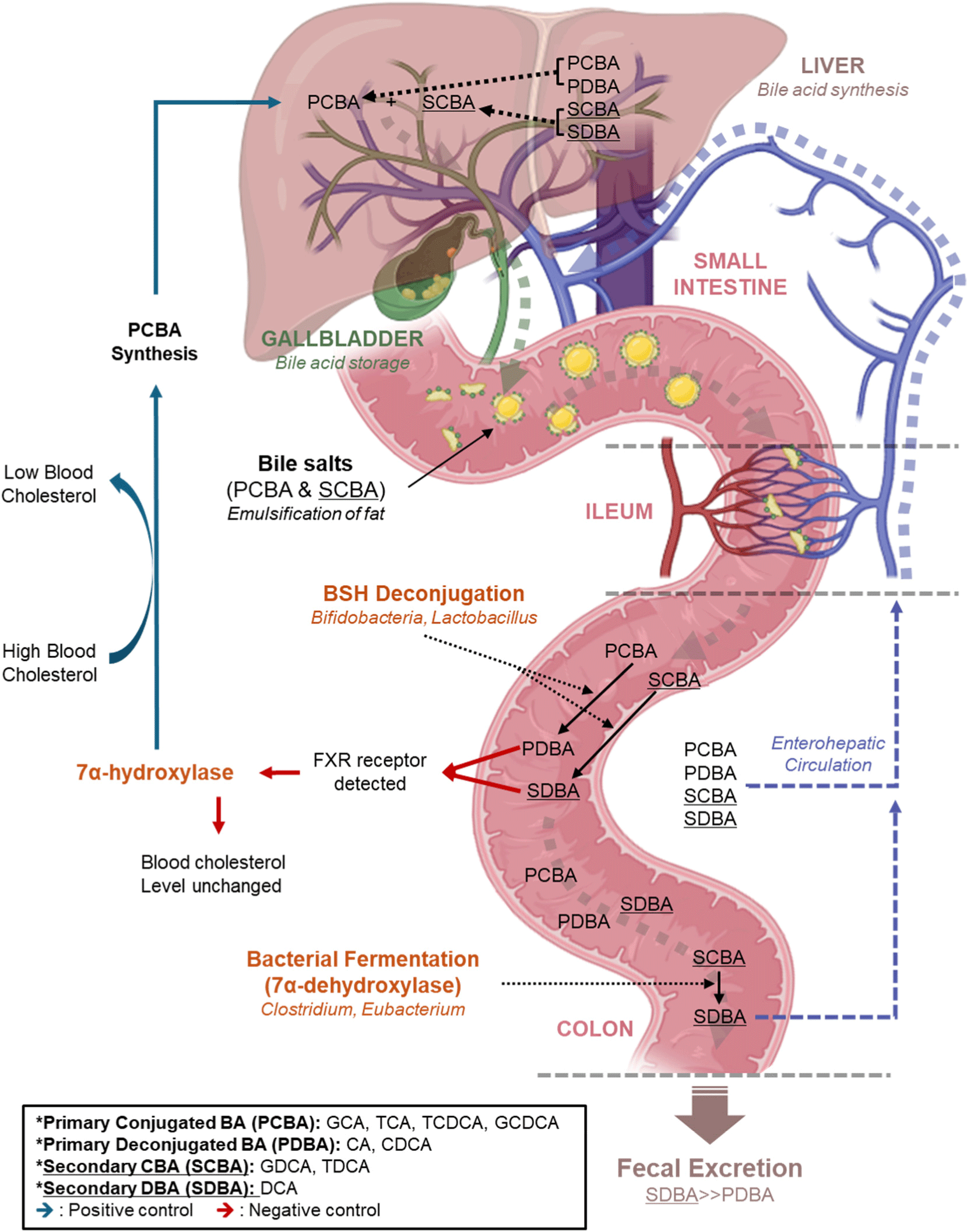
Several study reports revealed that high blood cholesterol levels (LDL-C>190 mg/dL; Bittencourt et al., 2020), accompanied by a sedentary lifestyle, are increasingly prevalent across generations (Evangelakos et al., 2021; Perez and Briz, 2009; Sun et al., 2023). Individuals with high plasma LDL-C levels have a high risk of developing CVD and a shorter life expectancy. Prescription of statins or ezetimibe to patients with high-cholesterol syndrome may relieve symptoms. However, they cause side effects, such as myopathy, acute pancreatitis, and liver toxicity (Florentin et al., 2008; Kashani et al., 2008). Furthermore, patients with liver or CVDs are particularly vulnerable to these side effects (Begley et al., 2006; Schuster, 2004).
Several studies have reported that probiotics can lower blood cholesterol levels with almost no side effects and have positive effects on various conditions, including the treatment of atopic dermatitis, colon cancer, Crohn’s disease, diarrhea, and constipation (Ishimwe et al., 2015; Ooi and Liong, 2010; Sivamaruthi et al., 2019). However, their respective mechanisms remain unclear (Gill and Guarner, 2004; Mercenier et al., 2003; Reid et al., 2003; Sanders and Klaenhammer, 2001; Tuohy et al., 2003; Woo et al., 2023). Ahn et al. (2003) reported that cholesterol was reduced following 4 weeks of consuming milk containing Lactobacillus acidophilus SNUL01, and Fuentes et al. (2013) highlighted that cholesterol was lowered by consuming the capsule form of Lactobacillus plantarum (CECT 7527, CECT 7528, and CECT 7529). The hypothesis supporting the reduction of blood cholesterol levels as a function of BSH revealed so far can be explained as follows: more than 95% of bile salts are reabsorbed in the human ileum (Li and Chiang, 2020; Naumann et al., 2020), and the remaining 5% passes through the ileum. Colonic bile salts are deconjugated by BSH activity, forming deconjugated bile salts. BSH has a specific active site (Fig. 2), especially the cys-2 (or 22) site, which is essential for BSH catalysis (Begley et al., 2006). In bile salt deconjugation, cys-2 attacks the carbonyl carbon of the excision amide bond in bile salt, followed by the removal of glycine or taurine by hydrolysis (Chand et al., 2018). Deconjugated bile salts are water-soluble in a colonic pH environment of 7–8 (Trivedi and Puranik, 2017; Yamamura et al., 2023). The metabolic activity of intestinal microorganisms, particularly lactic acid and short-chain fatty acid production, further lowers the pH, causing the precipitation of deconjugated bile salt (Begley et al., 2006). Therefore, colon enterocytes no longer absorb it, leading to its excretion in the feces, which in turn lowers blood cholesterol.
Nonetheless, confirming whether BSH is a factor remains difficult because microorganisms that reduce blood cholesterol levels exist despite the absence of the BSH gene, such as Streptococcus thermophilus MCC0200 (Kapse et al., 2024). In addition, Choi et al. (2015) reported that deconjugated bile salt has a stronger affinity for the farnesoid X receptor that regulates bile synthesis, reducing hepatic bile acid synthesis; by this result, the effect of BSH does not alter the blood cholesterol concentration. Consequently, current experimental results do not identify BSH activity as a major factor for the LDL-C-lowering effect in the presence of probiotics.
Even if probiotics do not have an LDL-C-lowering effect, maintaining high survival rates in the intestine is critical to elicit other health benefits to the host. This concept originated from the study of Fuller (1995). According to Dobson et al. (2012), probiotics are resistant to acid and produce antibacterial substances, including bacteriocins, hydrogen peroxide, and organic acids. Furthermore, probiotics are highly resistant to bile salts compared with other bacteria (da Silva et al., 2024; Gu et al., 2024; Horackova et al., 2020; Spínello et al., 2024; Urdaneta and Casadesús, 2017; Yang et al., 2019). The study by De Smet et al. (1995) suggested that BSH-positive probiotics would have stronger bile resistance than negative. However, subsequent studies showed no correlation between probiotic bile tolerance and BSH levels by enzyme knockout experiments (Begley et al., 2005; Moser and Savage, 2001). In addition, genomic analyses suggest no relationship between bile concentration and BSH gene expression (Horackova et al., 2020; Yang et al., 2019). Recently, Jarocki et al. (2014) hypothesized that deconjugated bile salt reacts with other organic substances, producing a hydrogel that can promote the colonization of intestinal microorganisms (Jarocki et al., 2014; Sobotka and Czeczowiczka, 1958). If these experiments can be replicated in vivo, new insights between BSH and probiotics can be processed.
Interspecies Characteristics of Bile Salt Hydrolase
The following data analysis is that which integrates the affinity between a single enzyme and bile. BSH activity has primarily been studied within the context of LAB research, and many full-length genomes of the strains studied have been identified. Begley et al. (2006) analyzed BSH activity mainly in Lactobacillus, Bifidobacterium, Enterococcus, Clostridium, and Bacteroides. However, most studies correlating enzyme activity with genomic data have used Lactobacillus species only (O’Flaherty et al., 2018). Considering that the taxonomy of Lactobacillus is newly defined, a new method for direct genetic analysis of the population is needed for comparison with other strains (Oberg et al., 2022). Furthermore, the current knowledge regarding the substrate affinity of BSH could enhance future genetic analyses of enzymatic mechanisms.
Table 1 summarizes the measured enzyme affinities for various BSH derived from different species. Notably, the taxonomy of Lactobacillus was recently updated as reported by Oberg et al. (2022). The affinity results of each BSH for conjugated bile salts (CA, DCA, and CDCA) were categorized to show the differences in affinity between glycine- and taurine-conjugated bile salts for each substrate. Higher affinity differences mean that an enzyme can hydrolyze a particular substrate faster or to a greater extent in a given data analysis method.
| Strain | Q1) | CA | DCA | CDCA | Reference |
|---|---|---|---|---|---|
| Lactobacillus | |||||
| L.acidophilus ATCC 4356 | 2 | - | Liong and Shah (2005) | ||
| L.acidophilus ATCC 4356-A | |||||
| L.acidophilus ATCC 4356-B | |||||
| L.acidophilus ATCC 4357 | 2 | + | Liong and Shah (2005) | ||
| L.acidophilus ATCC 4357-A | |||||
| L.acidophilus ATCC 4357-B | |||||
| L.casei ASCC 1521 | 1 | − | Liong and Shah (2005) | ||
| L rhamnosus strain ASCC 290 | 1 | + | Liong and Shah (2005) | ||
| L. casei ATCC 15820 | 1 | + | Liong and Shah (2005) | ||
| L. acidophilus LA1 | 2 | + | + | Kumar et al. (2012) | |
| L. acidophilus LA1-A | |||||
| L. acidophilus LA1-B | |||||
| L.fermentum K73 | + | +++ | Hernández-Gómez et al. (2021) | ||
| L.plantarum 299v | 1 | + | ++ | Hernández-Gómez et al. (2021) | |
| L.rhamnosus GG | 1 | + | +++ | Hernández-Gómez et al. (2021) | |
| L.johnsonii YB334 | + | − | Zhu et al. (2022) | ||
| L. plantarum Y14 | ++ | Liu et al. (2021) | |||
| L. paracasei subsp. X11 | − | Zhang et al. (2021) | |||
| L. acidophilus NCK 1909 | Foley et al. (2021) | ||||
| L. acidophilus NCK 1909-A | |||||
| L. acidophilus NCK 1909-B | |||||
| L. gasseri NCK2253 | Foley et al. (2021) | ||||
| L. gasseri NCK2253-A | −−− | −−− | −−− | ||
| L. gasseri NCK2253-B | +++ | +++ | +++ | ||
| L. paragasseri strain JCM 5343T | 3 | ||||
| L. paragasseri strain JCM 5343T-A | −− | + | − | Kusada et al. (2022a) | |
| L. paragasseri strain JCM 5343T-B | |||||
| L. paragasseri strain JCM 5343T-C | − | + | − | Kusada et al. (2022b) | |
| L. salivarius NRRL B-30514 | + | − | + | Wang et al. (2012) | |
| L. gasseri strain FR4 | + | + | Rani et al. (2017) | ||
| L. brevis ATCC 367 | 1 | ++ | ++ | +++ | Ru et al. (2019) |
| L.acidophilus NCDC291 | ++ | + | Kumar et al. (2012) | ||
| L. plantarum Lp91 | 1 | + | − | Kumar et al. (2012) | |
| L. plantarum WCFS1 | 4 | Lambert et al. (2008) | |||
| L. plantarum WCFS1-1 | +++ | +++ | +++ | ||
| L. plantarum WCFS1-2 | NA | NA | NA | ||
| L. plantarum WCFS1-3 | + | + | + | ||
| L. plantarum WCFS1-4 | +++ | +++ | +++ | ||
| L. rhamnosus strain E9 | 1 | + | + | + | Kaya et al. (2017) |
| L. fermentum NCDO394 | 1 | + | + | + | Kumar et al. (2013) |
| L. acidophilus PF01 | 1 | −−− | −−− | −−− | Oh et al. (2008) |
| L. johnsonii PF01 | 2 | Chae et al. (2013) | |||
| L. johnsonii PF01-A | −−− | −− | −− | ||
| L. johnsonii PF01-C | ++ | ++ | ++ | ||
| Bifidobacterium | |||||
| B.longum ATCC 15708 | + | + | + | Kim et al. (2004b) | |
| B. infantis KL 412 | + + | + + | + + | Kim et al. (2004b) | |
| B.suis NRRL B-41407 | 1 | + | + | + | Jarocki et al. (2014) |
| B.pseudocatenulatum DSM 20439 | 1 | + | + | + | Jarocki et al. (2014) |
| B.animalis subsp. lactis NRRL B-41405 | 1 | + + | Jarocki et al. (2014) | ||
| B.catenulatum DSM 20224 | 1 | + | Jarocki et al. (2014) | ||
| B.longum SBT2928 | + | + + | + | Tanaka et al. (2000) | |
| B.bifidum ATCC 11863 | 1 | + | + | + | Kim et al. (2004a) |
| B. animalis Bi30 | 1 | + + | + + | + + | Jarocki (2011) |
| B.longum BB536 | – | + | + | Grill et al. (1995); Li et al. (2021) |
|
| Enterococcus | |||||
| E. faecalis CU30-2 | 1 | + + + | + + + | + + + | Eom and Kim (2011) |
| E. faecalis T2 | 1 | + | + | + + | Chand et al. (2016) |
| E. faecium CRL183 | - | Taranto et al. (2000) | |||
| Bacteroides | |||||
| B. fragilis ATCC 25285 | 2 | + | + | + | Li et al. (2021); Stellwag and Hylemon (1976) |
| B. fragilis ATCC 25285-A | |||||
| B. fragilis ATCC 25285-B | |||||
Lactobacillus, Bifidobacterium, Enterococcus, and Bacteroides bile salt hydrolase information about G/T ratio by cholic acid, deoxycholic acid, and chenodeoxycholic acid affinity of BSH.
Table 1 only indicated the affinity between glycine-conjugated bile salt and taurine-conjugated bile salt for each BSH. Generally, the BSH data of Bifidobacterium and Enterococcus showed greater affinity for glycine-conjugated bile salts. In contrast, Lactobacillus showed varying affinities, and several strains harbored multiple copies of BSH genes (including L. acidophilus NCK 1909, Lactobacillus gasseri NCK2253, and Lactobacillus johnsonii PF01). These strains exhibited a higher affinity for taurine-conjugated bile salts.
Zhao et al. (2024) conducted a gene cloning experiment to heterologously express the BSH gene, explaining the relationship between probiotic bile acid affinity and the cholesterol-lowering effect, which specifically acts on either glycine- or taurine-conjugated bile salt. In addition, they administered them in mouse models to track their activity. This study reported that blood cholesterol levels decreased in post-germ-free mice carrying a mutant bacterium, F67A, that preferentially degrades taurocholic acid (TCA). However, the mutant bacteria, YB81, that preferentially degrades glycocholic acid (GCA), does not affect host blood cholesterol levels. In contrast, in specific pathogen-free mice, YB81 also reduced cholesterol levels. Therefore, the cholesterol-reducing mechanism of F67A involves altering the intestinal bile acid ratio, whereas YB81 lowers cholesterol levels by controlling the BSH activity of intestinal microorganisms. The results showed that YB81, which has a strong BSH affinity for glycine-conjugated bile salts in this case, changed the BSH activity of microorganisms in the gut in a way that does not directly lower cholesterol or have other metabolic effects. Lactobacillus fermentum K73, Lactobacillus rhamnosus GG, and Enterococcus faecalis CU30-2’s high affinity for glycine-conjugated bile salts might directly change the gut ecosystem compared with lower cholesterol based on Zhao’s hypothesis. In this regard, L. gasseri NCK2253 and L. johnsonii PF01 strains are important (Table 1). L. gasseri NCK2253-A and L. johnsonii PF01-A showed high affinities for the taurine-conjugated bile salt. L. gasseri NCK2253-B and L. johnsonii PF01-C BSHs showed opposite affinities (glycine-conjugated bile salts). Examining these two strains in vivo could provide more concrete evidence for the different substrate affinities of microbial BSH. Despite the abundance of data on BSH produced by Lactobacillus, additional studies on Bifidobacterium and Enterococcus are still needed to draw better comparisons of the BSH properties of different bacteria, particularly with the advent of tailored probiotics.
Potential Bile Salt Hydrolase Inhibitor for Feed Efficiency and Probiotics
Antibiotics are widely used in farms to improve domestic animal growth and maintain animal health. This phenomenon refers to antibiotics as AGPs (Lin, 2014). However, the use of antibiotics has caused the uncontrolled development of AMR in various niches. AMR is a driving force that promotes pools of resistant pathogenic bacteria and poses a serious threat to food safety and public health (Davies, 2014; Perry et al., 2014). For this reason, the incorporation of antibiotics in feed is legally restricted or completely banned in the EU, UK, USA, and other countries. Consequently, animal nutrition studies have focused on finding AGP alternatives and improving feed efficiency (Kim and Lee, 2005). To maximize feed efficiency, a substitution for antibiotics is necessary. The solution is yet to be determined, but currently, BSH control has the best consequences since suppressing BSH can achieve feed efficiency similar to that of using antibiotics.
Negga (2015) reported that BSH activity lowers blood cholesterol levels and feed efficiency. Furthermore, Rani et al. (2017) studied BSH inhibitors and found that riboflavin showed almost 98% inhibition. Notably, Negga hypothesized that riboflavin could increase the growth performance of domestic animals. Broiler chickens showed an increase in body weight after consuming 20 mg/kg of riboflavin for 21 days. Animal experiments using chickens and pigs proved that feeding vitamins, especially riboflavin, resulted in a similar level of increased productivity compared to that of antibiotic treatments (Geng, 2018; Negga, 2015; Yang et al., 2020). This phenomenon can be attributed to the functional inhibition of BSH by riboflavin and β-lactam antibiotics (penicillin V, ampicillin), especially penicillin (Adhikari et al., 2020; Daly et al., 2021; Geng and Lin, 2016; Li et al., 2022; Rani et al., 2017; Suresh et al., 1999). Given that the hypothesis is true, it is highly likely that BSH is the cause of the AGP effect. In order to achieve the AGP effect, an alternative to antibiotics, such as a BSH inhibitor, is required. However, the most effective treatments were limited to β-lactam antibiotics and riboflavin (Lin et al., 2014; Rani et al., 2017).
Therefore, to obtain sufficient feed effects from livestock, a biochemical mechanism and a more effective BSH inhibitor are necessary. For improved results, it is crucial to figure out the evolution of BSH and define the optimal binding site for the inhibitor. The following chapter presents the results of analyses based on the BSH peptide sequence and active site identified so far.
Bile Salt Hydrolase and Penicillin V Acylase Active Site and Mechanism of Action
BSH and PVA may be considered moonlighting proteins because of similar structures. Moonlighting proteins are defined as the same enzyme that performs more than one distinct action (Jeffery, 2018). However, the results of the experiment by Kumar et al. (2006) show that the enzymes presumed to be BSH or PVA from Bacillus sphaericus, Clostridium perfringens, and Bifidobacterium longum have only about 30% peptide similarity (not moonlighting protein). Otherwise, BSH and PVA are classified as choloylglycine hydrolases (CGH) within the N-terminal nucleophilic (Ntn) hydrolase enzyme superfamily (Daly et al., 2021).
These two enzymes, which appear to be similar only in structure, can hydrolyze each other’s substrates. There are genomic analyses for this phenomenon. O’Flaherty et al. (2018) reported that Lactobacillus gorillae, Lactobacillus frumenti, Lactobacillus vaginalis, Lactobacillus panis, Lactobacillus antri, Lactobacillus agilis, Lactobacillus salivarius, and L. plantarum strains are simultaneously active against bile acids and penicillin. For in vitro tests, Lambert et al. (2008) reported that L. plantarum WCFS1 has four bsh genes, including bsh-1, bsh-3, and bsh-4, which possess BSH activity. In contrast, bsh-2, bsh-3, and bsh-4 showed PVA activity, with bsh-3 showing the strongest activity. Furthermore, Kusada et al. (2022a) reported that L. paragasseri JCM 5343 bsh-A showed common substrate specificity for PVA.
However, because Lactobacillus was the primary focus of these results, it was necessary to compare strains belonging to Bifidobacterium or Enterococcus. For advanced data, phylogenetic analysis based on BSH peptide sequences obtained from the NCBI or ATCC databases was performed to determine the conserved domains of BSH. A phylogenetic tree was constructed using the data described in Table 2. The bacteria information source is based on Table 1 and searched against ATCC and NCBI databases. A Neighbor-Joining tree was constructed using the Jukes-Cantor model with uniform rates and bootstrap replications of 1,000 datasets using MEGA-11 software. Nodes farther apart are genetically distant, while genes on the same bridge are phylogenetically closer (Fig. 3).
1) The bacteria information source is based on Table 1 and searched against ATCC and NCBI databases. The Source ID starts with ATCC® is searched ATCC strain name using a search engine: https://www.atcc.org/?matchtype=&network=x&device=c&adposition=&keyword=&gad_source=1. The Source ID starts with the other searched strain name using a search engine: https://www.ncbi.nlm.nih.gov/.
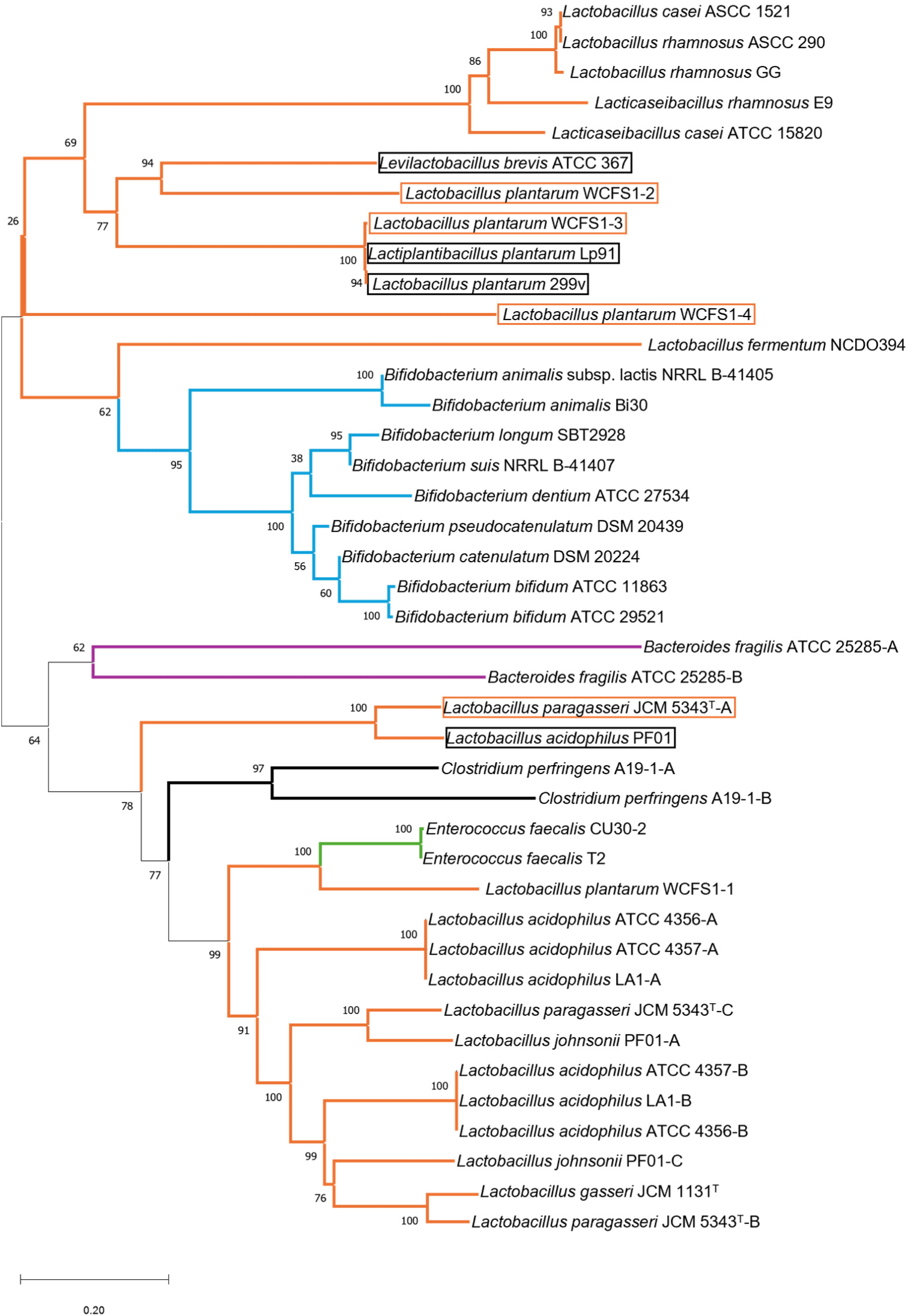
To explain Fig. 3, genes from Lactobacillus, Bifidobacterium, Enterococcus, Bacteroides, and Clostridium were represented using orange and blue lines. BSH genes that simultaneously or preferentially exhibit PVA activity were indicated using orange boxes, and BSH genes from strains that are highly likely to exhibit PVA activity were denoted using black boxes because they share the same bridge.
The reconstructed phylogeny suggests that Lactobacillus may have originated the BSH genes of Enterococcus and Bifidobacterium, in line with reports from Morinaga et al. (2022). Several clusters showed highly similar genetic distances from each other: Cluster 1 (Lactobacillus casei ASCC 1521, L. rhamnosus ASCC 290, and L.rhamnosus GG), and Cluster 2 (L. plantarum WCFS1-3, L. plantarum LP91, and L.plantarum 299V), and Cluster 3 (L.acidophilus ATCC 4356-A, L.acidophilus ATCC 4357-A, and L. acidophilus LA1-A), and Cluster 4 (L.acidophilus ATCC 4356-B, L.acidophilus ATCC 4357-B, and L.acidophilus LA1-B). The sequence identity percentage of the BSH gene was identical in each group. There is likely no affinity difference and the enzymes function similarly.
Table 1 shows differences in bile acid affinity within the same cluster, as revealed by the analysis. Within cluster 1, L. rhamnosus ASCC 290 and L. rhamnosus GG exhibited a strong affinity for GCA, whereas L. casei ASCC 1521 demonstrates a strong affinity for TCA. L. plantarum LP91, L. plantarum 299V and L. plantarum WCFS1-3 exhibited a strong affinity for GCA within cluster 2. However, L. plantarum 299V, and L. plantarum WCFS1-3 exhibited a strong affinity for GDCA, whereas L. plantarum LP91 demonstrates a strong affinity for TDCA. The BSH genes of L. acidophilus ATCC 4356, L. acidophilus ATCC 4357, and L. acidophilus LA1 in Cluster 3 exhibited a completely identical gene sequence with 100% similarity. Cluster 4 exhibited the same characteristics. Therefore, in either Cluster 3 or Cluster 4, the BSH should display identical affinities. However, comparing clusters 3 and 4 was challenging because an established BSH affinity for each substrate was lacking, as indicated in Table 1.
We conducted a comprehensive analysis to draw more conclusive interpretations of BSH and PVA activities from various LAB. Particularly with bsh genes that encode enzymes with PVA, such as L. paragasseri JCM 5343T-A and L. plantarum WCFS1-3, are important (Kusada et al., 2022a; Lambert et al., 2008). In addition, in Cluster 2, L. plantarum WCFS1-3, L. plantarum LP91, and L.plantarum 299V had highly similar nucleotide sequences. Therefore, it was necessary to determine whether LP91 and 299V can also metabolize penicillin.
The phylogenetic tree significantly correlated the BSH across the same species, as demonstrated in the previous chapter. It is important to analyze genetically connected, but the active site of these enzymes is also important. The active site is predicted using point mutations as explained in Chand et al. (2018). Most active sites reported for BSH appear to be highly conserved.
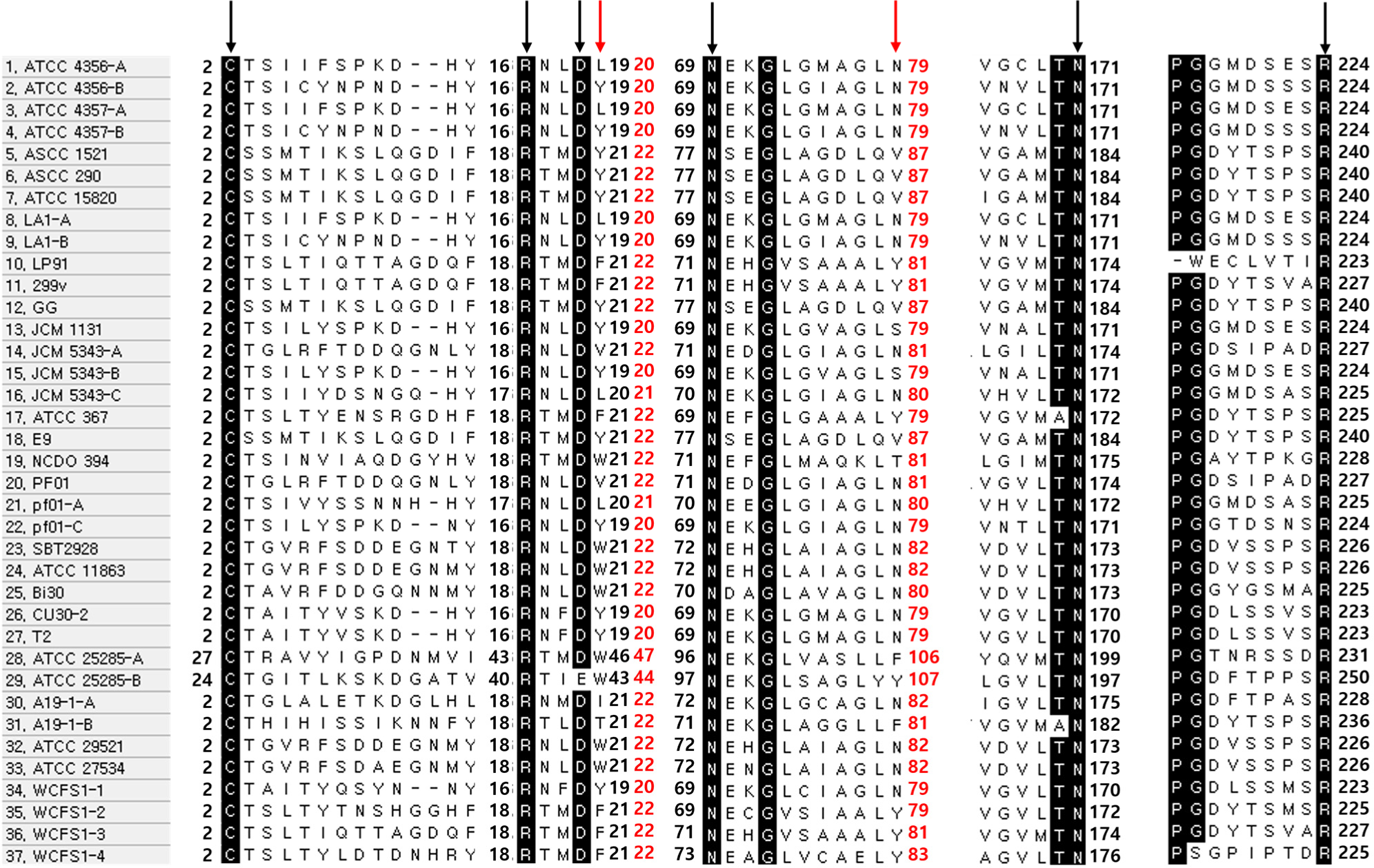
To determine whether this fact appears not only in Lactobacillus but also in Bifidobacterium or Enterococcus, sequence alignment was performed based on the active site. Chand et al. (2018) confirmed the active site of BSH by using point mutations in a predicted region. Most active sites reported for BSH appear to be highly conserved. In B. bifidum, Cys-2, Arg-18, Asp-21, Asn-72, Asn-173, and Arg-226 are predicted to be the residues involved in active sites (Kim et al., 2004a; Song et al., 2019). Regarding this, the BSH gene sequence was aligned and compared with the peptides described above using MEGA. Except for Bacteroides fragilis ATCC 25285-B, which changed Asp-21 to Glu-43 as shown in Fig. 2, all samples shared the same active site.
These results confirm the homogeneity of almost all active sites, making it difficult to distinguish between PVA and BSH based on this aspect. Finding the difference between the two is very important for understanding the identity and mechanism of BSH in the future. Meanwhile, Avinash et al. (2016) reported that two Trp residues (at positions 23 and 87, based on B. bifidum) of PVA were important for interactions having the benzene ring of penicillin. In addition to Trp, Phe, and Tyr (benzene ring amino acids) were discovered in PVA’s peptide sequence of PVA (Chand et al., 2018; Daly et al., 2021; Suresh et al., 1999).
Based on this theory, Fig. 2 is analyzed additionally. The bsh-A gene of L. paragasseri strain JCM 5343T and the second, third, and fourth bsh genes of L. plantarum WCFS1 showed experimentally verified PVA activity. The 2nd, 3rd, and 4thbsh genes in L. plantarum WCFS1 have conserved Phe-23 and Tyr-87 residues. In contrast, the first bsh gene in L. plantarum WCFS1 did not exhibit PVA activity and contained only one benzene ring amino acid (Tyr-22 and Asn-87). However, the bsh-A gene of L. paragasseri strain JCM 5343T contains Val-23 and Asn-87 except for the benzene ring amino acid, with an affinity for ampicillin (Kusada et al., 2022a). These results make it difficult to conclude that the amino acids at positions 23 and 87 of the benzene ring are critical for PVA activity. Therefore, further research is vital and required to determine which amino acid sequence produces PVA activity.
If BSH is intended for use in medication, postbiotics (cell-free supernatants and soluble factors secreted by live bacteria; Martyniak et al., 2021) or an overexpression protocol can be used without the genetic manipulation of probiotics. However, postbiotics safety has not yet been verified (Zhong et al., 2023). Also, BSH has unknown risks proved by in vitro and in vivo analyses.
Several studies reported that DCA, in reaction to BSH, can be a causal factor for CRC. Analysis revealed that patients with CRC had high levels of secondary bile acids in their large intestine (Aguirre et al., 2022; Choi et al., 2015; Sun et al., 2023). Ajouz et al. (2014) reported that excessive concentration of secondary bile acids that pass into the large intestine may cause inflammatory responses, cell membrane destruction, and DNA damage. This affects the intestinal cells, ultimately leading to CRC.
Another potential risk is that BSH may act as an antibiotic resistance factor, given that both BSH and PVA belong to the CGH family. In this regard, investigation of the active site or peptide sequence holds little relevance in distinguishing BSH with PVA activity. The PVA enzyme inhibits penicillin activity, allowing bacteria to survive in the presence of the said antibiotics (Lambert et al., 2008; Sunder et al., 2017). Kusada et al. (2022a) reported that L. paragasseri JCM 5343Tbsh-A has AMR by BSH activity. Furthermore, Lambert et al. (2008) reported that L. plantarum WCFS1 bsh-2, bsh-3, and bsh-4 showed PVA activity, with bsh-3 showing the strongest activity. However, these phenomena were observed mostly in vitro.
Until now, only the negative effects of secondary bile acids produced by BSH have been highlighted. Studies have indicated that certain intestinal diseases are caused by an imbalance of secondary bile acids. Diversity of intestinal microorganisms is needed for a healthy BSH pool, which therefore balances the secondary bile acids. In several studies, patients with inflammatory bowel disease (IBD) had significantly reduced amounts of secondary bile acids, DCA and LCA (Fiorucci et al., 2021; Heinken et al., 2019; Larabi et al., 2023). Ultimately, the key is to prevent excessive formation of secondary bile acids.
Complete inhibition of BSH activity would eliminate its cholesterol-lowering effect in the blood, which would be a disadvantage in various aspects. Instead, preventing the conversion of primary deconjugated bile salt to secondary deconjugated bile salt could effectively maintain an appropriate amount of primary and secondary bile acids. Bustos et al. (2018) reported that the 7α-dehydroxylase of gut bacteria removes the 7α-hydroxy group and converts primary deconjugated bile salts to secondary deconjugated bile salts (Fig. 1). However, LAB do not have this function. According to Takahashi and Morotomi (1994), bacterial genera used as probiotics (bifidobacteria and lactobacilli) cannot dehydrogenate primary deconjugated bile salts. Thus, if we can control the 7α-dehydroxylation pathway, we will also be able to suppress the formation of excessive secondary bile acids.
To summarize, the effects of BSH need to be studied further for safe use. While the suppression of BSH generally has positive effects, it can also lead to various side effects, underscoring the importance of mitigating methods to control the 7-dehydroxylation pathway.
Future Research
Multiple BSH genes and their surrounding regions showed minimal sequence similarity, indicating that BSH is the product of horizontal gene transfer. Furthermore, insertion into similar regions and the existence of mobile genetic markers support this theory (Daly et al., 2021). The PVA enzyme inactivates penicillin activity, allowing bacteria to survive in the presence of antibiotics (Lambert et al., 2008; Sunder et al., 2017). BSH and PVA are difficult to distinguish based on structural or peptide sequence differences because BSH and PVA share about 30% sequence similarity with each other, but in fact, about 30% similarity is also found among BSHs across different species (Kumar et al., 2006). Also show different substrate specificities for each strain (Lambert et al., 2008). Understanding the causes of these characteristics is important for future gut microbiome research.
Many studies have examined the correlation between IBD and CRC, the amount of BSH, and the proportion of bile acid (Evangelakos et al., 2021; Fiorucci et al., 2021; Heinken et al., 2019; Larabi et al., 2023; Perez and Briz, 2009; Sun et al., 2023). However, it was only found that representative microorganisms produced active BSH and did not measure the colonic pH of healthy people and patients or sufficiently investigate the composition of LAB. A recent study measured the real-time colonic pH and showed that the normal cecal pH is approximately 5.5, and the large intestine is approximately pH 5.5 to pH 7 (31, 32). If the production of deconjugated bile salt occurs at the beginning of the large intestine, the pH at which it precipitates sufficiently matches this. Therefore, further research is needed on changes in the ratio of intestinal LAB, colonic pH, and the toxicity of deconjugated bile salt.
Recently, a published paper showed that BSH with different substrate specificities towards glycine- and taurine-bile acids has differences in how the microorganism regulates cholesterol (Zhao et al., 2024). This study explains the reason for the difference in bile salt affinity, which was previously difficult to interpret solely based on the BSH sequence. However, the lack of relevant papers necessitates sufficient verification, allowing us to directly design cholesterol control mechanisms in patients or healthy individuals using probiotics.
To date, most studies on BSH have focused on those involving LAB, especially Lactobacillus. However, understanding the relationship between BSH and PVA, the evolutionary history of BSH and PVA, and the biological flow of genes requires a deeper understanding of the relationship between BSH and various microorganisms. Therefore, in addition to Lactobacillus, Bifidobacterium, and Enterococcus, research on the BSH of Listeria, Clostridium, and other microorganisms is required.
Conclusion
Compared to other blood cholesterol-reducing drugs, the body naturally uses BSH as part of food consumption, which makes it commercially valuable. Understanding the risks, functions, and characteristics of BSH can further ensure the safety of probiotics, which can directly impact the intestinal survival rate. Therefore, it is important to clarify the cause of the characteristic strains with substrate specificity and measure the pH of the patient’s colon who suffered from IBD or CRC.













