Introduction
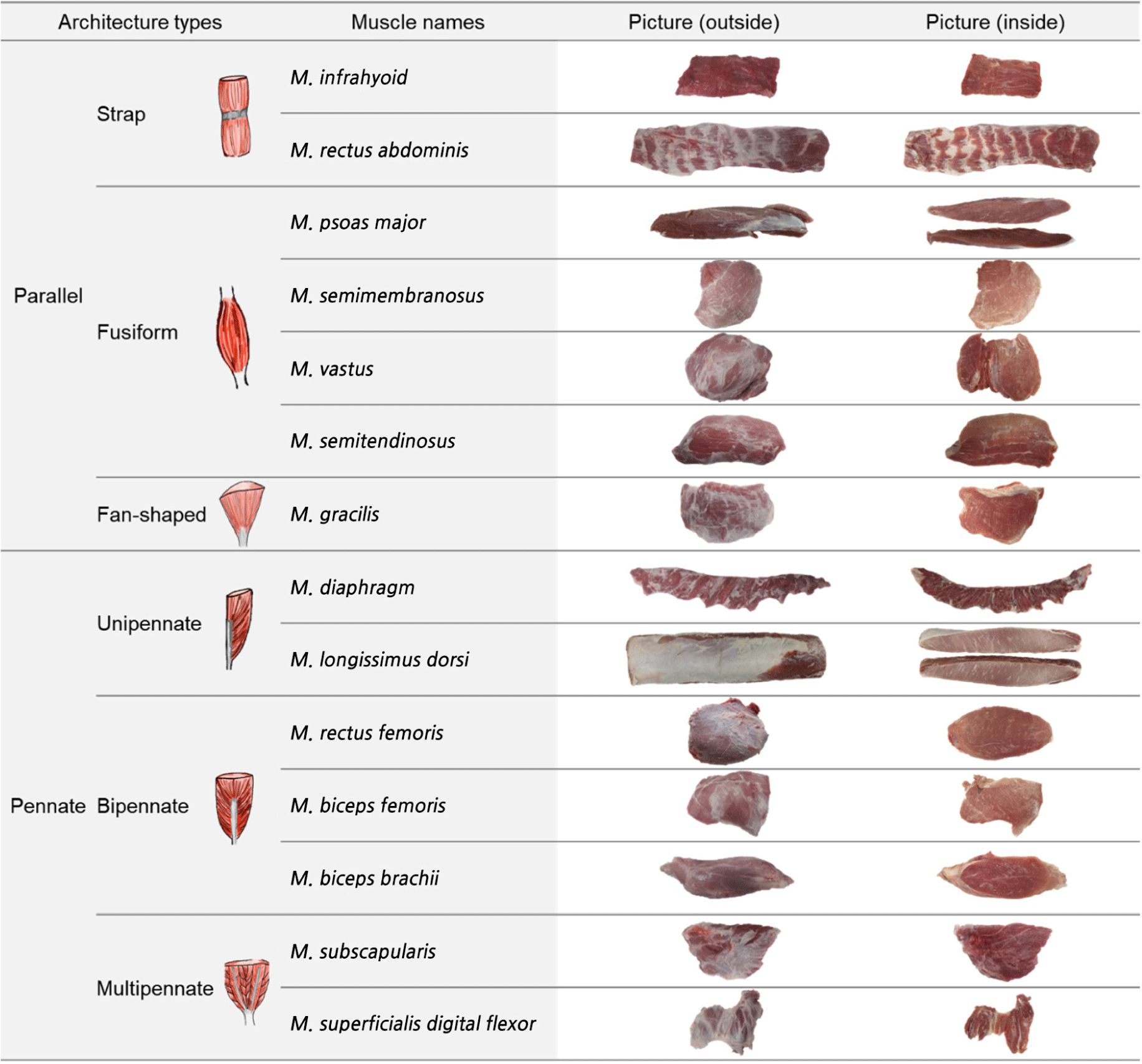
The skeletal muscle is a structure formed by the systematic arrangement of muscle fibers, connective tissues, and tendon elements (Roy and Edgerton, 2009). These structural features control the transmission of the force generated by the muscle-tendon complex and the movement of each organ and tissue (Woittiez et al., 1984). The morphology of each muscle depends on its physiological and mechanical functions. Muscles are classified according to their morphologies (Lieber and Fridén, 2000; Narici, 1999), with the two main types being parallel and pennate, where the muscle fibers are parallel or at an angle to the force-generating axis, respectively (Wilson, 2014). These two types are further differentiated into strap (string or belt-like shape), fusiform (wider and cylindrical in the center and tapering at the ends), or fan-shaped (a form in which fibers converge at on end) for the parallel type, and unipennate (the shape in which the muscle fibers are oriented at one fiber angle to the force generating axis), bipennate (a form with fibers on both sides of the tendon), and multipennate (a form in which fibers are composed of several tendons in various directions) for the pennate type (Wilson, 2014).
The function of a muscle is to generate contraction and force to move or resist. The length and angle of muscle fibers depend on the architecture of the muscle (Klont et al., 1998; Lieber, 2002; Woittiez et al., 1984). Each muscle is composed of several types of muscle fibers with the contractile, metabolic, and physiological properties of each muscle differing according to their fiber characteristics (Armstrong et al., 1987; Totland and Kryvi, 1991). Muscle fiber characteristics are closely related to the physicochemical properties of meat, regardless of the animal species (Choi and Kim, 2009; Joo et al., 2013; Karlsson et al., 1999; Kim et al., 2018; Ozawa et al., 2000; Ryu and Kim, 2005). Various studies have reported the following results regarding the relationship between muscle fiber characteristics and physicochemical properties of meat: (1) The relative compositions of type I and IIA fibers are positively correlated with redness and water-holding capacity, while those of type IIB fibers are negatively correlated with water-holding capacity and tenderness (Joo et al., 2013; Kim et al., 2018); (2) Muscles composed of a large amount of type I fibers have a high intramuscular fat content (Karlsson et al., 1999); (3) Type IIA and IIB fibers show positive and negative correlations, respectively, with the ultimate post-mortem pH (Ryu and Kim, 2005); (4) Depending on the muscle type, the muscle fiber characteristics are different, which means that the meat quality characteristics of each cut are different. In both porcine and bovine muscles, the M.psoas major (PM; tenderloin) consists of relatively smaller muscle fibers and larger amounts of type I and IIA fibers compared to the M.longissimus dorsi (LD; loin), the M.semimembranosus (SM; round), and the M.semitendinosus (ST; eye of round) (Cheng et al., 2020; Cheng et al., 2021; Hwang et al., 2010; Kim et al., 2016). Accordingly, the tenderloin generally has better water-holding capacity, tenderness, and color than other cuts, regardless of the species (Joo et al., 2013).
The relationship between meat quality and muscle fiber type differences has been well established in previous studies. However, studies on the physicochemical properties and muscle fiber characteristics of muscles according to morphological properties in terms of meat science are scant. Therefore, this study aimed to investigate the differences in physicochemical and muscle fiber characteristics of porcine skeletal muscles with different morphologies (muscle architecture).
Materials and Methods
Porcine skeletal muscles were obtained from the left side of crossbred (Landrace×Yorkshire×Duroc, castrated) pig carcasses (n=9, 79.2±3.5 kg carcass weight) at 24 h post-mortem, at a commercial slaughterhouse. According to the muscle architectures, 14 types of muscles including M.infrahyoid (IF), M.rectus abdominis (RA), M.gracilis (GR), PM, ST, SM, M.vastus (VT), M.diaphragm (DP), LD, M.biceps brachii (BB), M.biceps femoris (BF), M.rectus femoris (RF), M.subscapularis (SS), and M.superficialis digital flexor (SDF) were obtained from each carcass and classified into two main groups, parallel and pennate. The muscles were further subdivided into subtypes (parallel: strap, fusiform, and fan-shaped; pennate: unipennate, bipennate, and multipennate), as shown in Fig. 1. For the analysis of proximate composition, meat quality properties, and muscle fiber characteristics, the central part of each muscle was collected. Samples for immunohistochemical staining were prepared using the Guth and Samaha (1969) method, with slight modifications. Briefly, muscle pieces (1.0×1.0×1.5 cm) were cut from the central region of each muscle and immediately frozen in 2-methylbutane cooled by liquid nitrogen. Frozen samples were kept at –80°C until staining. The remaining part of each sample was used for the analysis of proximate composition (e.g., moisture, crude fat, crude protein, and crude ash) and meat quality properties (e.g., pH, drip loss, cooking loss, meat color, and shear force).
The proximate composition data were collected in triplicate for each sample. Moisture and crude ash were measured using the AOAC (2000) method, with slight modifications. To analyze the moisture content of the samples, 5.0 g was dried in an oven at 105°C for 12 h. To measure crude ash, approximately 2 g of the sample was pre-dried at 105°C for 4 h and then burned at 600°C for 6 h. The weights of the dried and burned matter were expressed as percentages of the initial weight of the sample for the moisture and crude ash content, respectively. The Kjeldahl method (AOAC, 2000) was used to determine the crude protein content. Briefly, 0.5 g of the sample was digested using sulfuric acid, and the ammonia distilled into boric acid. The borate anions were titrated with hydrochloric acid and the crude protein content expressed by multiplying the analyzed total nitrogen content by 6.25. Crude fat content was determined according to Folch et al. (1957), with slight modifications. Briefly, the 5.0 g of the sample was homogenized in 35 mL of Folch solution (chloroform:methanol, 2:1, v:v) and placed in the refrigerator (4°C) for 2 h. Next, the homogenate was filtered through a filter paper (Whatman No. 1, Merck KGaA, Darmstadt, Germany), and a 0.88% NaCl solution used to separate the filtrate into two layers. The upper layer was removed, and the crude fat collected after evaporating the lower layer using nitrogen gas. Crude fat was expressed as a percentage of the sample.
Data for meat quality properties were collected in triplicate for each muscle. For pH determination, 3 g of each sample was homogenized with 27 mL of deionized water, and the pH of the homogenate measured using a pH meter (S220, Mettler Toledo, Greifensee, Switzerland). To evaluate the meat color of the porcine skeletal muscles, the muscles were cut and exposed to air for 20 min to oxygenate the myoglobin. Meat color was measured using a colorimeter (CR-400, Konica Minolta, Tokyo, Japan) after calibration with a white plate (Y=93.5, x=0.3132, y=0.3198). The meat color was presented according to the Commission Internationale de l’Eclairage system (CIE, 1977): lightness, CIE L*; redness, CIE a*; and yellowness, CIE b*. To determine the water-holding capacity of the samples, drip and cooking losses were measured. Drip loss was determined by analyzing the weight loss after suspension of the muscle (approximately 50 g) in a plastic bag for 24 h, according to Honikel (1987), with slight modifications. Cooking loss was determined by placing 40 g of the sample in a plastic bag and cooking it in a water bath (75°C) until the internal temperature reached 70°C. After cooling to room temperature for 30 min, the weight loss was measured. Drip loss and cooking loss were expressed as percentages of the initial weight of the muscle pieces before suspension and cooking, respectively. To determine the tenderness of porcine skeletal muscles, muscle cores (1.0 cm in diameter) were obtained from the cooked samples by removing them parallel to the muscle fiber orientation. The cores were sheared vertically using a Warner-Bratzler shear blade in a texture analyzer (TA1, Ametek, Largo, FL, USA), and the shear force values (N/cm2) recorded.
To evaluate the muscle fiber characteristics of porcine skeletal muscles, the muscle fibers were stained using immunohistochemistry according to Song et al. (2020), with slight modifications. Briefly, transverse sections (10 μm) were collected from each muscle using a cryostat microtome (CM1520, Leica Biosystems, Wetzlar, Germany) at –20°C. The sections were subjected to the following staining procedure: Blocking in 10% normal goat serum (Cell Signaling Technology, Danvers, MA, USA); incubation with primary antibodies (BA-D5, SC-71, BF-35, and BF-F3, DSHB, Iowa City, IA, USA) specific to myosin heavy chain (MHC) isoforms; and incubation with secondary anti-IgG and anti-IgM antibodies conjugated with fluorescent dyes (Alexa Fluor 488, 505, and 594, Thermo Fisher Scientific, Waltham, MA, USA). The sections were visualized using a fluorescence microscope (EVOS M5000, Thermo Fisher Scientific). Three different parts per section were captured, and approximately 600 muscle fibers identified. Muscle fibers were classified into six types according to the distribution of MHC isoforms as follows: I, MHC I/slow; IIA, MHC 2a; IIAX, MHCs 2a and 2x; IIX, MHC 2x; IIXB, MHCs 2x and 2b; IIB, MHC 2b. Muscle fiber characteristics, including the cross-sectional area (CSA; μm2), relative fiber area (%), and fiber density (number/mm2), were analyzed using an Image Pro Plus Program (Media Cybernetics, Rockville, MD, USA).
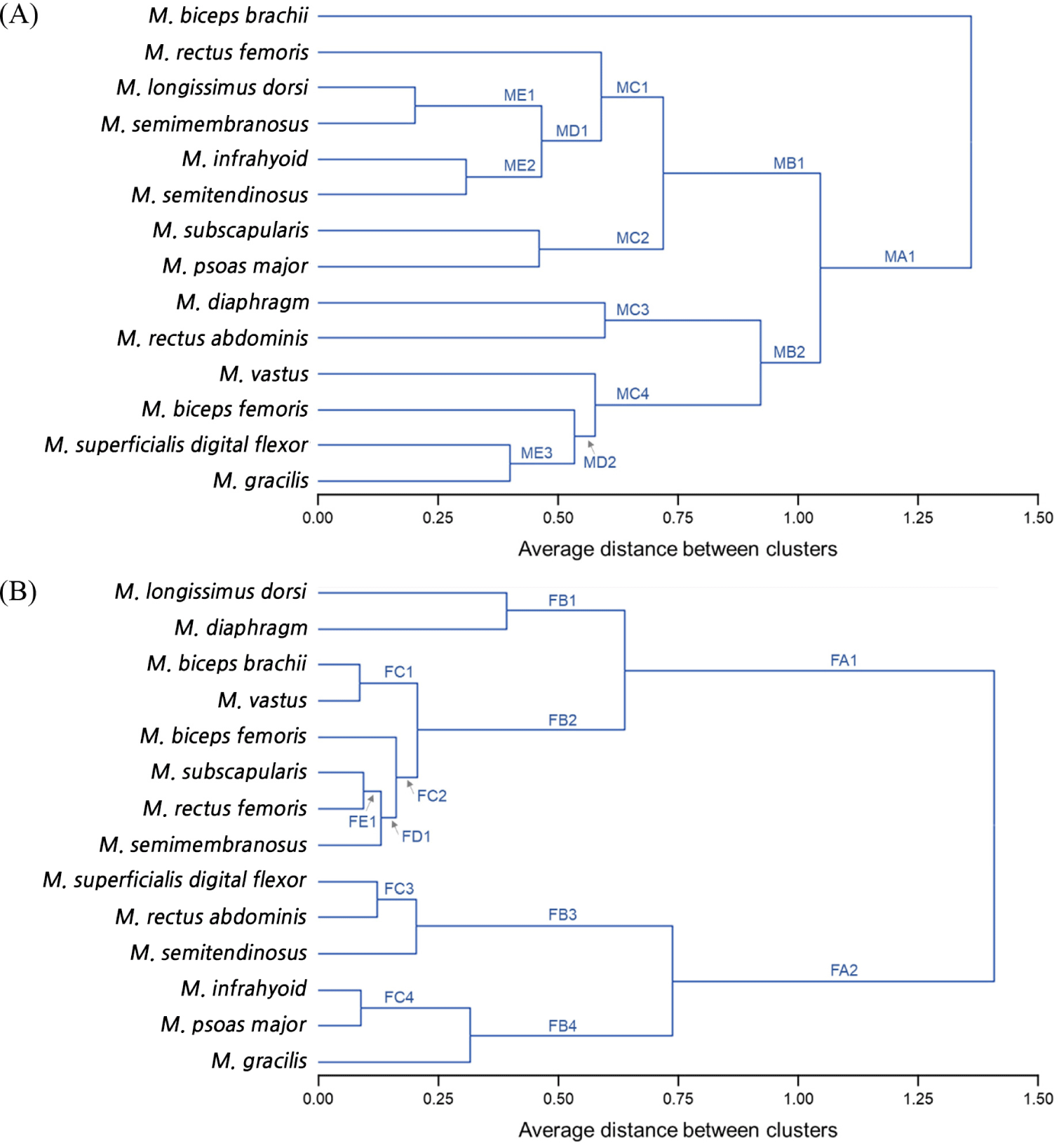
For each muscle of the 14 types obtained from each pig carcass, samples were collected at 3 regions and each measurement repeated three times per sample (technical repeats). The data are expressed as the average±SE for the pooled muscle architecture groups. Data were analyzed using the SAS software (ver. 9.4, SAS Institute, Carry, NC, USA). Normal distribution and equal variance of the data were verified using PROC UNIVARIATE. To compare proximate compositions, meat quality properties, and muscle fiber characteristics between muscles within the same muscle subtypes, between muscle structural subtypes within the same muscle architecture groups, and between parallel and pennate groups, the Student’s t-test and a one-way ANOVA were performed. A post-hoc test between groups showing a significant difference was conducted using Duncan’s multiple range test. Differences were considered statistically significant at p<0.05. To analyze the similarities between the 14 types of porcine skeletal muscles, a hierarchical cluster analysis (HCA) was conducted. The Euclidean distance and similarity index by meat quality properties were 0.20 (high similarity) to 1.36 (low similarity), and for muscle fiber characteristics, it was found to be 0.14 (high similarity) to 1.32 (low similarity).
Results
The proximate composition, pH, meat color, water-holding capacity, and shear force of each muscle from the parallel types are shown in Table 1. Among the subtypes of muscle architecture, the fan-shaped group consisted of only GR. For fusiform, four types of muscles (PM, ST, SM, and VT) showed significant differences in moisture content, meat color, water-holding capacity, and shear force (p<0.05). More specifically, ST showed a lower moisture content and higher lightness, cooking loss, and shear force compared to PM and VT (p<0.05). However, the moisture content, lightness, yellowness, and shear force of ST were not significantly different from those of SM. All measurements, except for yellowness and cooking loss, were not significantly different between the PM and VT groups. In the strap group, significant differences were observed in pH, redness, and shear force between IF and RA (p<0.05), while the other traits (e.g., proximate composition, lightness, redness, and water-holding capacity) were not significantly different between the two muscle types.
As shown in Table 2, muscles grouped according to pennation (uni-, bi-, and multi-pennation) showed a significant difference in pH within the same subtype of muscle architecture (p<0.05). The unipennate group showed significant differences in most physicochemical properties (p<0.05), except for crude ash, lightness, and water-holding capacity. LD muscle had higher moisture, crude protein, and shear force but lower fat content, pH, redness, and yellowness than DP (p<0.05). In the bipennate group, BF had a lower moisture content, higher crude fat content, and higher yellowness than BB and RF (p<0.05). A similar trend was observed for most physicochemical properties, except for lightness, redness, and cooking loss, between BB and RF. SS and SDF, which are clustered as multipennate types, were not significantly different in all physicochemical properties except for pH, drip loss, and shear force. The pH and shear force were higher, and the drip loss lower, in SS compared to SDF (p<0.05).
The results of the comparison of physicochemical properties between subtypes within the same architecture, and between parallel and pennate architectures, are shown in Table 3. The strap type had a lower moisture content and a higher redness than the fan-shaped and fusiform types (p<0.05). The fan-shaped subtype had significantly higher crude fat and pH, but lower yellowness, than the fusiform subtype (p<0.05). Of the pennate subtypes, the unipennate subtype had a lower moisture content than the other pennate subtypes (p<0.05), but the highest lightness (p<0.05). The bipennate subtype did not show significant differences in pH and redness with the unipennate subtype, and moisture content, lightness, and redness with the multipennate subtype.
Muscle fiber types I, IIA, IIAX, IIX, IIXB, and IIB were detected in most of the muscles, however, type IIAX fibers were not detected in ST, IF, DP, and LD. In addition, type IIB fibers were not distributed in the IF (Fig. 2; Tables 4 and 5). The muscle fiber characteristics of the parallel type muscles are presented in Table 4. Here, PM had the largest fibers, regardless of muscle fiber type, among the fusiform-shaped muscles. The mean CSA was higher in the PM muscle than in the ST, SM, and VT muscles (p<0.001). Relative fiber area of type I was lowest in ST, while those of types IIA and IIXB were lowest in SM. In addition, SM comprised 51.0% of type IIB which was significantly higher than PM (10.7%) and VT (25.4%; p<0.001). Regarding fiber density, significant differences among the fusiform-shaped muscles were observed regardless of the muscle fiber type (p<0.05). Specifically, the fiber density of type I was the lowest in ST, while that of types IIAX, IIX, and IIB was significantly lower in PM compared to the other types (p<0.05). The lowest type IIB fiber density was observed in the SM muscle (p<0.05). Two strap-shaped muscles, IF and RA, showed significant differences in the CSA of types IIA, IIXB, and the mean CSA value (p<0.05). Except for types IIAX and IIB, which were not detected in IF, all types of muscle fibers were numerically larger in IF than in RA. For relative fiber area, only type IIA showed a significant difference between IF and RA, which was higher in IF (p<0.001). However, fiber density did not show any significant differences between IF and RA, regardless of muscle fiber type.
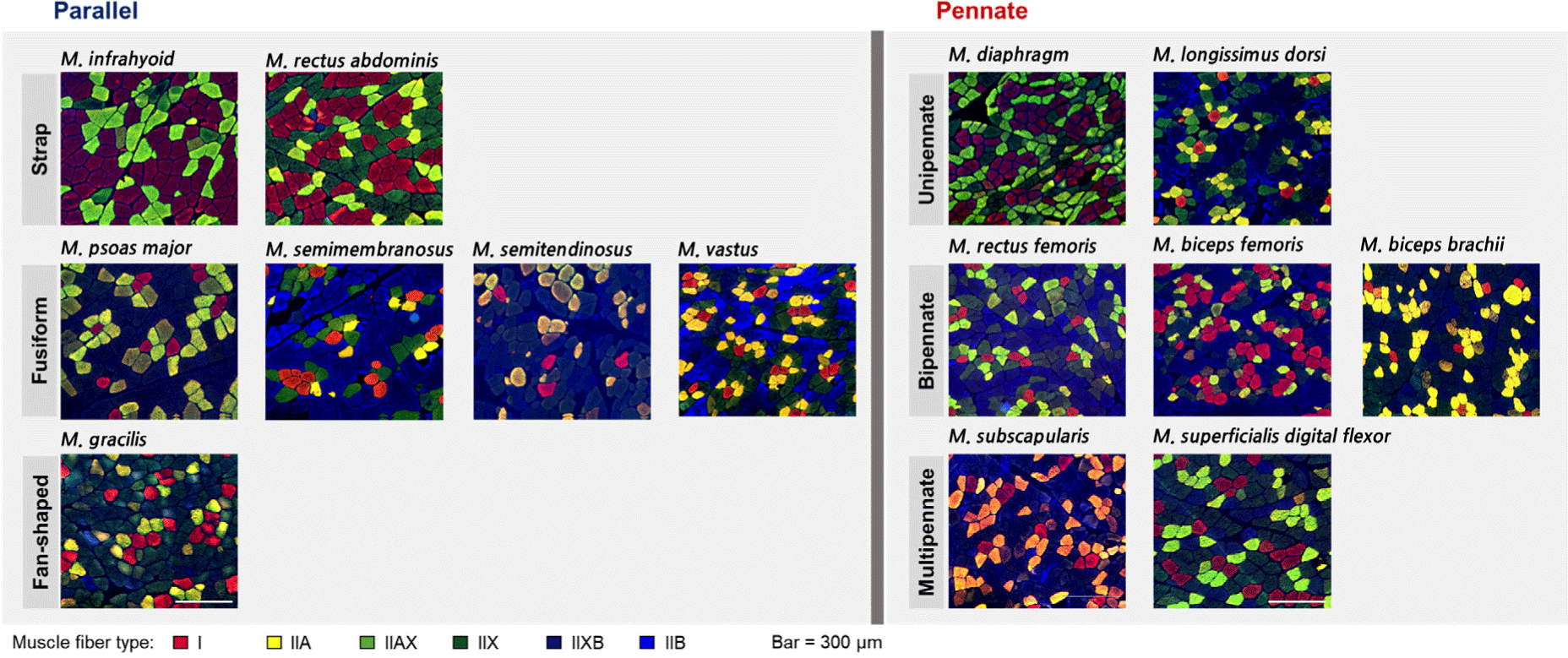
The muscle fiber characteristics of the pennate subtype are shown in Table 5. The CSA of type IIA was greater in DP than in LD (p<0.01). Other types did not show significant differences in CSA between DP and LD (p>0.05). The relative fiber area and fiber density of types I, IIA, IIX, and IIB showed the same trend in DP and LD. For example, the relative fiber area and fiber density of types I and IIA were higher in DP than in LD, whereas those of types IIX and IIB were lower in DP than in LD (p<0.05). The muscles (BB, BF, and RF) grouped into the bipennate subtype were not significantly different in the CSA of all types of muscle fibers. However, the relative fiber areas of types IIAX and IIX were significantly different between the muscles (p<0.05) and were higher in BB than in BF and RF. Regarding fiber density, BB consisted of higher IIX fibers but lower IIB fibers than BF and RF (p<0.05). The fiber density of type IIA was the lowest in the BF (p<0.05). For the multipennate subtype, SS and SDF had different sizes of IIX, IIXB, and IIB fibers. In addition, these fibers were significantly larger in SS than in SDF (p<0.05). Although there were no significant differences in the CSA of types I, IIA, and IIAX, the mean CSA was higher in SS than in SDF (p<0.01). The relative fiber area did not show significant differences for any muscle fiber type. Types IIA fiber density and the total number of fibers were higher in SDF than in SS (p<0.05), whereas there were no significant differences in other types of muscle fibers.
In the muscles with parallel architecture, the fan-shaped group had larger fibers of type IIA, IIAX, and IIB than the fusiform group (p<0.05; Table 6). The strap group had smaller IIAX and IIB fibers than the fan-shaped group (p<0.05), however, the CSA of the other types did not differ between the strap and fan-shaped groups (p>0.05). In addition, the fiber size of the strap group was not significantly different from that of the fusiform group in all fiber types, except for type IIAX. A similar trend was observed in the relative fiber area and fiber density between the parallel architecture subtypes. Specifically, these two characteristics of type I were higher in the strap group, whereas those of type IIX were higher in the fan-shaped group than in the other subtypes (p<0.05). However, the relative fiber area and fiber density of type IIB were higher in the fusiform group than in the fan-shaped and strap groups (p<0.05). In the pennate architecture, the multipennate group had larger fibers than the unipennate group, regardless of the fiber type (p<0.05). In addition, all types of fibers, except for type IIB, were larger in the multipennate group than in the bipennate group (p<0.05). The unipennate group contained a higher amount of IIA fibers, but a lower amount of IIX fibers, than the other subtype groups (p<0.05). The fiber density showed that more type I and IIA fibers were distributed in the unipennate group than in the bipennate and multipennate groups (p<0.05). Moreover, the total number of fibers per unit area was highest in the unipennate group (p<0.05). When comparing the muscle fiber characteristics between the parallel and pennate architectures, most types of fibers (I, IIA, IIX, and IIXB) were larger in the parallel than in the pennate architectures (p<0.05). However, no significant differences were observed in the relative fiber areas of all fiber types between the parallel and pennate architectures. The fiber density of type IIA and total fiber number were higher in the pennate than in the parallel structures (p<0.05).
Based on the similarity of the physicochemical properties and muscle fiber characteristics, the muscles were clustered by HCA (Fig. 3). The similarity in physicochemical properties between muscles was the highest (1.36) between BB and the other muscles (MA1; Fig. 3A). Except for BB, two or more muscles were clustered by Euclidean distance between 0.20 and 1.36. Specifically, LD and SM were grouped with 0.20 of Euclidean distance (ME1). These muscles were further grouped with IF and ST (ME2). These two groups showed similarities with RF and MC2 (SS and PM) and clustered into the same group (MB1). The other main group consisted of six muscles (e.g., DP, RA, VT, BF, SDF, and GR; MB2). Within the MB2 group, the SDF and GR were linked (ME3) and further grouped with the BF (MD2). The MD2 group showed a similar Euclidean distance to VT and was clustered into MB2 with DP and RA (MC3). The results of HCA by muscle fiber characteristics showed a different trend from that of the physicochemical properties (Fig. 3B). Here, LD was grouped with DP (FB1), while BB was grouped with VT (FC1). In addition, SS was linked to RF (FE1) and further grouped with SM (FD1). Moreover, BF showed the same Euclidean distance as FD1 and was categorized into the same group with FC1 (FB2). Within the FA1 group, FB1 and FB2 were grouped, which is distinguished from the FA2 group (consisting of SDF, RA, ST, IF, PM, and GR). Within the FA2 group, SDF was linked to RA (FC3) and grouped with ST (FB3). The FB3 group was distinguished from the FB4 group by the presence of FC4 (IF and PM) and GR.
Discussion
Various studies have looked at the effect of muscle type on physicochemical properties and muscle fiber characteristics. Bee et al. (2004) demonstrated that muscle fiber composition is different between LD, RF, and ST. Further, studies conducted by Cheng et al. (2020) and Hwang et al. (2010) showed that PM had higher oxidative fibers (I and IIA) than other muscles (LD, SM, or ST). In the present study, similar results were observed in the muscle fiber compositions of these muscles. Specifically, the muscle fiber composition of PM was distinct from that of LD, RF, SM, and ST. It was expected for PM to be different from LD and RF in muscle fiber characteristics due to its different morphological structures (parallel vs. pennate). However, the low similarity in muscle fiber composition between PM, SM, and ST was unexpected since they are the same subtype (fusiform). These results suggest that muscle fiber composition is determined by the intrinsic function of individual muscles rather than by their architecture. Although muscle architecture is mainly associated with mechanical function (Sutherland et al., 1980; Zajac, 1989), individual muscles are in different regions of the body and play different roles in movement and force generation (Davies, 1981; Ward et al., 2009).
In addition to the muscle mechanical function, muscle fiber characteristics are influenced by the force performance (resistance vs. endurance) and energy dependence (oxidative vs. glycolytic) of individual muscles (Farup et al., 2014; Lieber, 2002). In the present study, DP (76.2%), IF (82.6%), and RA (69.2%) comprised relatively higher amounts of oxidative fibers (I and IIA) than the other muscles, ranging from 15.4% to 52.4%. Conversely, LD (84.5%), ST (78.5%), SM (76.2%), GR (70.6%), VT (69.3%), and RF (62.5%) comprised over 60% of the glycolytic fibers (IIX, IIXB, and IIB). Except for the strap and fusiform of parallel architecture, it seems that the muscle fiber compositions (relative area) of other subtypes of parallel and pennate architectures, were not highly related to the morphological properties of the muscles.
In this study, pennate type muscles were mainly composed of smaller fibers than parallel type muscles. Moreover, the unipennate group showed the smallest muscle fiber size compared to the bipennate and multipennate groups. Pennate-fibered muscles have an advantage in force generation with the same size of muscle as parallel-fibered muscles because there are more fibers in the pennate than in the parallel architectures (Maclntosh et al., 2006). In addition, the fiber density was higher in the pennate architecture than in the parallel architecture. Therefore, these results indicate that muscle fiber size and density are closely related to force generation and muscle fiber orientation.
Species, breed, sex, genotype, and muscle type are the main factors that influence muscle fiber characteristics concerning meat quality (Cheng et al., 2020; Joo et al., 2013; Karlsson et al., 1999; Maltin et al., 1997; Monin and Ouali, 1992; Pette and Staron, 1990; Ryu and Kim, 2005; Waritthitham et al., 2010). In the present study, the strap muscles and DP, composed of a large number of oxidative fibers, where relatively redder than the other muscles. Previously, it was reported that muscles consisting of a high amount of oxidative fibers showed higher pH, redness, tenderness, and water-holding capacity than those composed of large amounts of glycolytic fibers (Choi et al., 2007; Joo et al., 2013; Kim et al., 2010; Ryu and Kim, 2005). However, the present study did not show consistencies in these meat quality traits, except for redness. The muscles with high compositions of glycolytic fibers did not align with the results of previous studies. Accordingly, these results show similar trends to those of muscle fiber characteristics, especially muscle fiber composition, suggesting that physicochemical properties are not significantly affected by muscle architecture.
The HCA results supported the above suggestions that the similarities (Euclidean distances) were low between muscles within the same subtype of both parallel and pennate architectures. That is, muscles with the same morphology have the same type of force generation and mechanical properties. However, the specific role and function differ depending on the location, intensity of exercise, and the type of force performance (resistance vs. endurance) (Totland and Kryyi, 1991).
Conclusion
Although muscle architecture is determined by mechanical and physiological functions, muscles with the same architecture (morphological properties) do not have the same muscle fiber characteristics and physicochemical properties as porcine skeletal muscles. The different trends in muscle fiber composition and meat quality properties were caused by the types of muscles playing different roles in different positions, rather than by muscle architecture. In addition, these results indicate that in meat industry, quality control is necessary by considering the physicochemical and histological characteristics of each muscle regardless of the muscle architecture.













