Introduction
Cultured meat represents a promising alternative protein source, with the potential to closely mimic the texture and nutritional value of conventional meat. This is achieved through in vitro cultivation of muscle and other types of animal cells, which are fundamental components of muscle tissue. The successful culture of muscle stem cells (MuSCs) hinges on four critical factors: the selection of appropriate cell types, the formulation of cell culture media, the design and composition of scaffolds, and the scalability of mass production processes (Lee et al., 2023). These elements are deeply interconnected, each influencing the outcomes of cultured meat production.
Among various cell types, MuSCs have been predominantly used for cultured meat research because the skeletal muscle constitutes the main parts of meat, despite the potential of fibroblasts, pre-adipocytes, and smooth muscle cells in cultured meat applications (Pasitka et al., 2023; Yuen et al., 2023; Zheng et al., 2022). The choice of culture media is pivotal, with a significant focus on reducing or completely eliminating fetal bovine serum (FBS) to improve sustainability and ethical considerations in bovine MuSC cultures (Kolkmann et al., 2022; Stout et al., 2022; Stout et al., 2023). Furthermore, differentiation media that do not rely on FBS or serum have been developed, highlighting advancements in cell culture techniques (Messmer et al., 2022). Mass cultivation is a process that is not easily achieved in academic or general research institutions; it is a focus more prevalent within the industrial sector due to its complexity and the resources required. Then, the remaining factor is the scaffold for cultured meat.
The development and optimization of scaffolds, which provide the necessary structural support for cell attachment and growth are crucial. In the cultured meat industry, hydrogels, microcarriers, and porous scaffolds are widely used, necessitating careful adjustment of properties such as hydrophilicity/hydrophobicity, charge, and physical characteristics (e.g., stiffness, elasticity, size, shape, topography) to mimic the extracellular matrix (ECM) effectively (Bodiou et al., 2020; Lee et al., 2023). While gelatin, collagen, or RGD-peptide are commonly employed due to their cost-effectiveness and edibility, they may not be the most suitable ECM components for cultivating porcine MuSCs, as suggested by prior studies (Samandari et al., 2023). The tissue microenvironment, or ‘niche,’ which includes the ECM comprising collagens, fibronectins, and laminins plays a vital role in cellular behavior, affecting cell adhesion, migration, proliferation, differentiation, and apoptosis (Chowdhury et al., 2015; Thomas et al., 2015).
Laminin, in particular, stands out due to its significant role in supporting cell adhesion and differentiation, offering unique advantages for muscle tissue engineering (Penton et al., 2016). Its ability to closely mimic the natural muscle tissue environment makes it a potentially superior choice for the cultivation of porcine MuSCs. This study aims to compare the effects of different ECM proteins, including collagen, fibronectin, gelatin, and laminin, on the culture of porcine MuSCs. Despite previous comparisons of these ECM components (Dodson et al., 1990; Wilschut et al., 2010), the direct impact of laminin on the proliferation and differentiation rates crucial for enhancing cultured meat productivity warrants thorough investigation.
Therefore, this study focused on evaluating the effectiveness of various ECM coatings to facilitate the in vitro culture of porcine MuSCs, with a particular emphasis on optimizing culture conditions for improved cell proliferation and differentiation. The insights gained from this study are expected to significantly contribute to optimizing MuSC culture on two-dimensional plates and guiding future scaffold-based research in cultured meat production, particularly highlighting the potential of laminin in advancing muscle tissue engineering.
Materials and Methods
This study was conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) of Seoul National University, bearing the approval code SNU-180612-2. The experimental procedures strictly complied with the guidelines set by the Institute of Laboratory Animal Resources at Seoul National University. The MuSCs donor for this study were four female Berkshire piglets, 14 days old, procured from DaSan Jongdon, located in Namwon, Republic of Korea.
Tissue culture plates were coated with commercial ECM proteins diluted in their respective solvents: 0.1% porcine gelatin (w/v; G1890, Sigma-Aldrich, St. Louis, MO, USA), 0.1% fibronectin (w/v; 10838039001, Sigma-Aldrich), and 0.05% laminin (w/v; 11243217001, Sigma-Aldrich) were diluted in Dulbecco’s Phosphate-Buffered Saline (DPBS; Welgene, Gyeongsan, Korea), while 0.1% collagen (w/v; 11179179001, Sigma-Aldrich) was diluted in 0.2% acetic acid solution (v/v; Duksan Pure Chemical, Ansan, Korea). The coatings were applied for 1 hour in a 37°C incubator. Subsequently, the residual solutions were carefully removed before cell seeding.
Porcine MuSCs were obtained from four 14-day-old female piglets, following the method outlined by Choi et al. (2020a). The biceps femoris muscles of the piglets were harvested post-euthanasia using CO2 inhalation followed by exsanguination. These muscles were initially cleansed using DPBS with a 2× concentration of antibiotic-antimycotic solution from Gibco (AA; Gaithersburg, MD, USA), after which any connective, vascular, and fat tissues were removed. The tissues were then minced, enzymatically digested with 0.8 mg/mL Pronase (Sigma-Aldrich) for 40 min at 37°C, with vortexing every 10 min. After digestion, the tissues were spun down at 1,200×g for 15 min. The resulting suspension was then mixed in Dulbecco’s Modified Eagle’s Medium (DMEM; Welgene) with 10% FBS (Gibco), to separate undigested material, and this suspension was centrifuged at 300×g for 5 min, filtered through a 100 μm cell strainer, followed by another centrifugation at 1,200×g for 15 min to collect cell pellets. Before purification using magnetic-activated cell sorting (MACS), cells were cryopreserved in a solution comprising 90% DMEM (supplemented with 10% FBS and 1× AA) and 10% dimethyl sulfoxide (DMSO; Mylan, Canonsburg, PA, USA) and then stored in liquid nitrogen.
For the purification of MuSCs, we employed MACS using a Cluster of Differentiation 29 (CD29) antibody, as detailed in a prior study (Choi et al., 2020a). In brief, the thawed cells were cultured on gelatin-coated dishes in GM (SkGM-2 medium, Lonza, Basel, Switzerland), supplemented with 20 μM SB203580 (Cayman Chemical, Ann Arbor, MI, USA) and 1× AA). Once the cells reached around 90% confluence, they were dissociated using 0.04% Trypsin-EDTA (Gibco). The cells were then mixed with CD29 antibody (MAB17783, R&D Systems, Minneapolis, MN, USA) at a 1:100 ratio and anti-mouse IgG microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) at a 1:5 ratio. Magnetic labeling and separation were performed using an MS column (Miltenyi Biotec) following the manufacturer’s protocol.
The sorted porcine MuSCs were seeded onto above mentioned ECM-coated dishes at the density of 5×104 cells/well of 12-well plate and cultured with GM. The cultured cells were collected from the next day to third day after subculture and fixed with 4% paraformaldehyde. The number of cells were counted using a cell counter (ScepterTM 2.0 Cell Counter; Merck Millipore, Darmstadt, Germany).
On the third day of proliferation, MuSCs at 90% confluency were used for myogenic differentiation. The cells were cultured in a differentiation media consisting of DMEM containing 2% (v/v) horse serum (Biowest, Nuaillé, France), 1× GlutaMAX (Gibco), 1× AA, and 1× β-mercaptoethanol (Gibco) for 2 days without media changes. After myofiber formation from the MuSCs was evident, the cells were fixed with 4% paraformaldehyde or treated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) for further analysis.
Cultured cells were pre-incubated for 10 min at 4°C and fixed in 4% (w/v) paraformaldehyde for 30 min. After washing twice with DPBS, the samples were permeabilized with 0.2% (v/v) Triton X-100 (Sigma-Aldrich) for 15 min and blocked with 10% (v/v) goat serum in DPBS for 1 hour. Then, the cells were incubated with primary antibody recognizing myosin heavy chain (MHC; 1:1,000; MAB4470, R&D Systems) overnight at 4°C. After washing three times with 0.1% TWEEN®20 (Sigma-Aldrich) in DPBS, the cells were incubated with the appropriate Alexa Fluor-conjugated secondary antibody (Invitrogen) overnight at 4°C. The nuclei were stained with Hoechst 33342 (Molecular Probes, Eugene, OR, USA). Images of stained cells were captured using an inverted fluorescence microscope (Eclipse TE2000-U, Nikon, Konan, Japan). The differentiation ratio was calculated by the proportion of the MHC-positive cells with the total number of cells.
Total RNA from cultured cells was extracted using TRIzol reagent (Invitrogen), and cDNA synthesis was performed using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA). The cDNA amplification was carried out with a DyNAmo HS SYBR Green quantitative real-time polymerase chain reaction (qPCR) Kit (Thermo Fisher Scientific) containing 1–2 pmol of each primer set (Table 1) in a 10 μL reaction volume. Amplification and detection were performed using the ABI 7300 Real-Time PCR System (Applied Biosystems) following the conditions one cycle at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension for 1 min (60°C for GAPDH, MYF5, MYOD1, MYOG, MYH1, MYH2, MYH4, MYH7, PAX7). The dissociation curves were analyzed. Relative expression levels were calculated by normalizing the threshold cycle (Ct) values of each gene to the reference gene, GAPDH, using the delta-delta Ct method.
An analysis using the general linear model was conducted by SAS software (version 9.4, SAS Institute, Cary, NC, USA) with four biological replicates unless otherwise specified. To identify notable distinctions among the groups, Turkey’s multiple range test was utilized, setting the statistical significance at a p-value less than 0.05.
Results and Discussion
To evaluate the effect of various ECM coatings on the proliferation of porcine MuSCs, the number of cells was counted over three days (Fig. 1). Initially, on day one, there were no significant differences in the number of MuSCs, their morphology, or confluence across the groups (Fig. 1A). All groups exhibited the elongated morphology typical of proliferative myoblasts (Fu et al., 2015). By day two although cell counts did not differ significantly, cells on laminin-coated surfaces began to show distinct cell alignment, indicating that MuSCs on laminin had already started initial differentiation to myotubes (Fig. 1A). On day three, cell counts in laminin-coated dishes were significantly higher than those in other groups (Fig. 1B, p<0.05), indicating a preferential boost in MuSC proliferation on laminin through enhanced cell alignment. Meanwhile, MuSCs on other coatings started to show cell alignment to a lesser extent compared to laminin. This finding is crucial for cultured meat production, as it links laminin coatings directly to increased productivity through higher proliferation rate and more pronounced initial differentiation than other coatings. Laminin emerges as a prime candidate for MuSC proliferation coatings, echoing the findings of Öcalan et al. (1988) with murine myoblasts who also noted faster proliferation on laminin compared to collagen and fibronectin coatings. Chowdhury et al. (2015) observed that human myoblasts on laminin proliferated faster than on plain dishes, and also noted that fibroblasts proliferated more slowly than myoblasts on laminin.
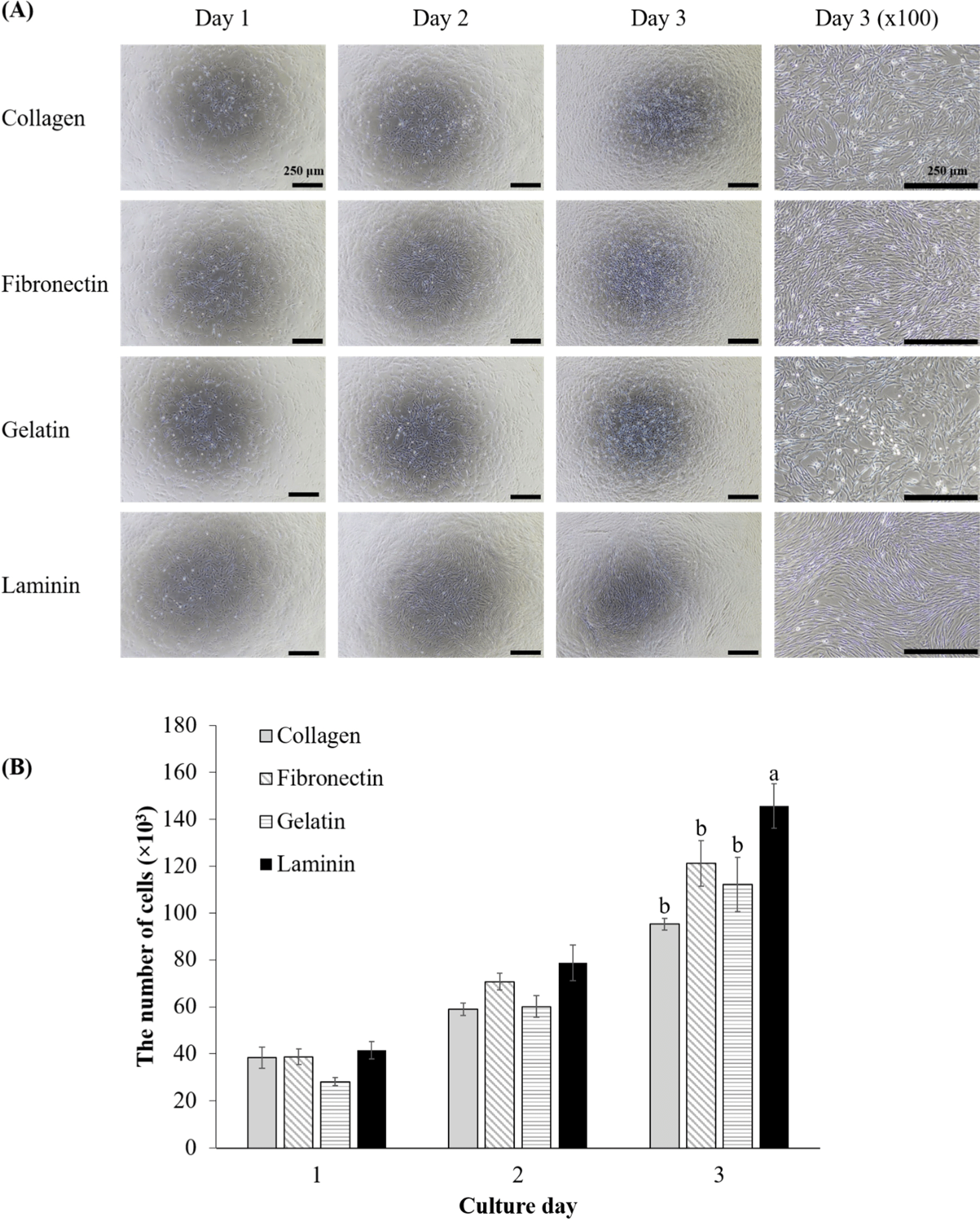
Other studies have reported that fibronectin and laminin effectively support the attachment of porcine MuSCs compared with collagen, gelatin, and Matrigel (Wilschut et al., 2010), and fibronectin was the best attachment materials for ovine MuSCs, while gelatin was the best inducer for ovine MuSCs proliferation among ECM coating materials including Cell-Tak®, fetuin, fibronectin, gelatin, laminin, poly-L-lysine, poly-D-lysine, and type I and IV collagens (Dodson et al., 1990). However, in our study, no differences in attachment were observed among ECM coating materials, as inferred by the lack of difference in cell numbers on day 1. Cultured meat research often utilizes animal-derived proteins for coatings or scaffolds to provide attachment sites for cells, typically employing collagens or gelatins from bovine, porcine, and fish sources (Samandari et al., 2023). However, the results may indicate that these collagens and gelatins are not the best options for the proliferation of porcine MuSCs. This study highlights the potential of laminin as a preferred ECM coating for future applications, aiming to induce proliferation of MuSCs. Consequently, given that porcine MuSCs demonstrated enhanced proliferation on laminin-coated dishes compared to others, further investigation was conducted for ECM`s impact on early myogenic gene expression through mRNA analysis.
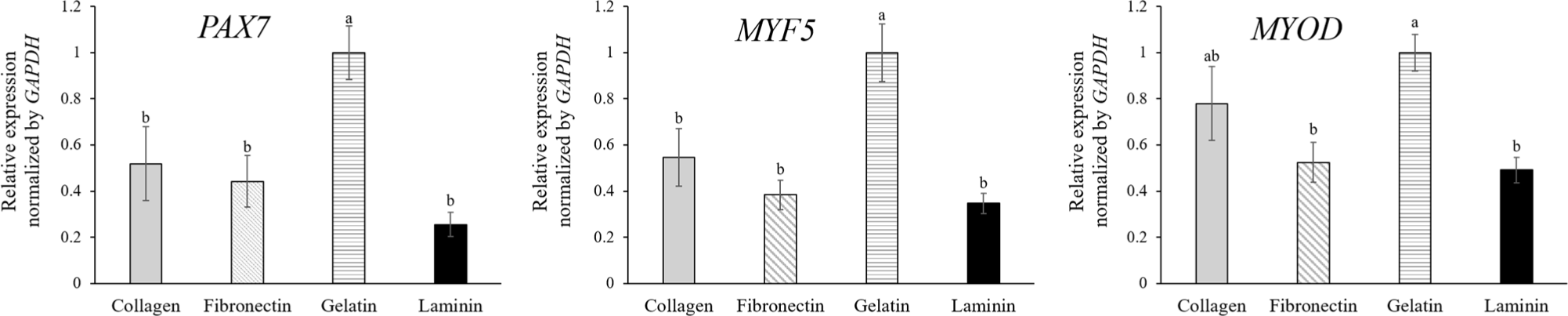
The effect of ECM coatings on MuSC gene expression revealed differential expression levels of PAX7, MYF5, and MYOD. In their dormant state, satellite cells are characterized by the expression of PAX7 while lacking the expression of MYF5 and MYODin vivo (Choi et al., 2020b). Upon muscle damage, these satellite cells commit to a myogenic pathway, evidenced by the simultaneous upregulation of MYF5 and MYOD and a concurrent decrease in PAX7 expression, triggering the cells’ progression from quiescence to active proliferation (Choi et al., 2020b; Kim et al., 2023). The in vitro culture of MuSCs mirrors the muscle repair process, which is why these specific markers are instrumental for the analysis of MuSCs. As myoblasts continue to develop, PAX7 expression ceases, which signals the onset of terminal differentiation to muscle fiber (Jin et al., 2016). PAX7, a crucial regulator of MuSCs (Ding et al., 2017), is nearly universally expressed by dormant satellite cells and is found alongside MYOD in proliferating myoblast progeny (Collins et al., 2009). However, our study showed that MuSCs cultured on gelatin-coated dishes exhibited higher gene expression on the third day of proliferation compared to other ECM coatings (Fig. 2A) despite a lower proliferation rate (Fig. 1B). This may indicate that cells on gelatin are less activated, possibly because cells on other coatings had reached confluency, initiating early differentiation before serum starvation. The lack of correspondence with cell numbers suggests another factor, such as fibroblast contamination, which is known to adhere more readily to collagen-coated and plain dishes than myoblasts (Chowdhury et al., 2015). Although the cells were isolated using MACS, yielding a purity of 91.5±2.40% as verified in our previous study (Choi et al., 2020a), the possibility remains that fibroblast remnants could proliferate more rapidly on gelatin. This could explain the observed cell proliferation results, which showed similar confluency to cells on other ECM coatings. Additionally, our results suggest that interactions between ECM coatings and MuSCs may differ from those reported in previous literature. Wilschut et al. (2010) showed that porcine MuSCs from newborn piglets had higher expression levels of PAX7 and MYF5 when cultured on laminin and fibronectin coatings than collagen, gelatin, and Matrigel, and MuSCs on laminin-coated dish formed clusters. Conversely, our study showed that MuSCs on laminin proliferated faster than those on collagen, fibronectin, and gelatin without clustering and expressed lower PAX7 compared to those on gelatin, with similar expression levels to those on collagen and fibronectin. Therefore, predicting myogenic potential based solely on mRNA expression from MuSCs in proliferation proves challenging. Consequently, we analyzed the myogenic potential of myoblasts on different ECM-coated dishes by inducing differentiation.
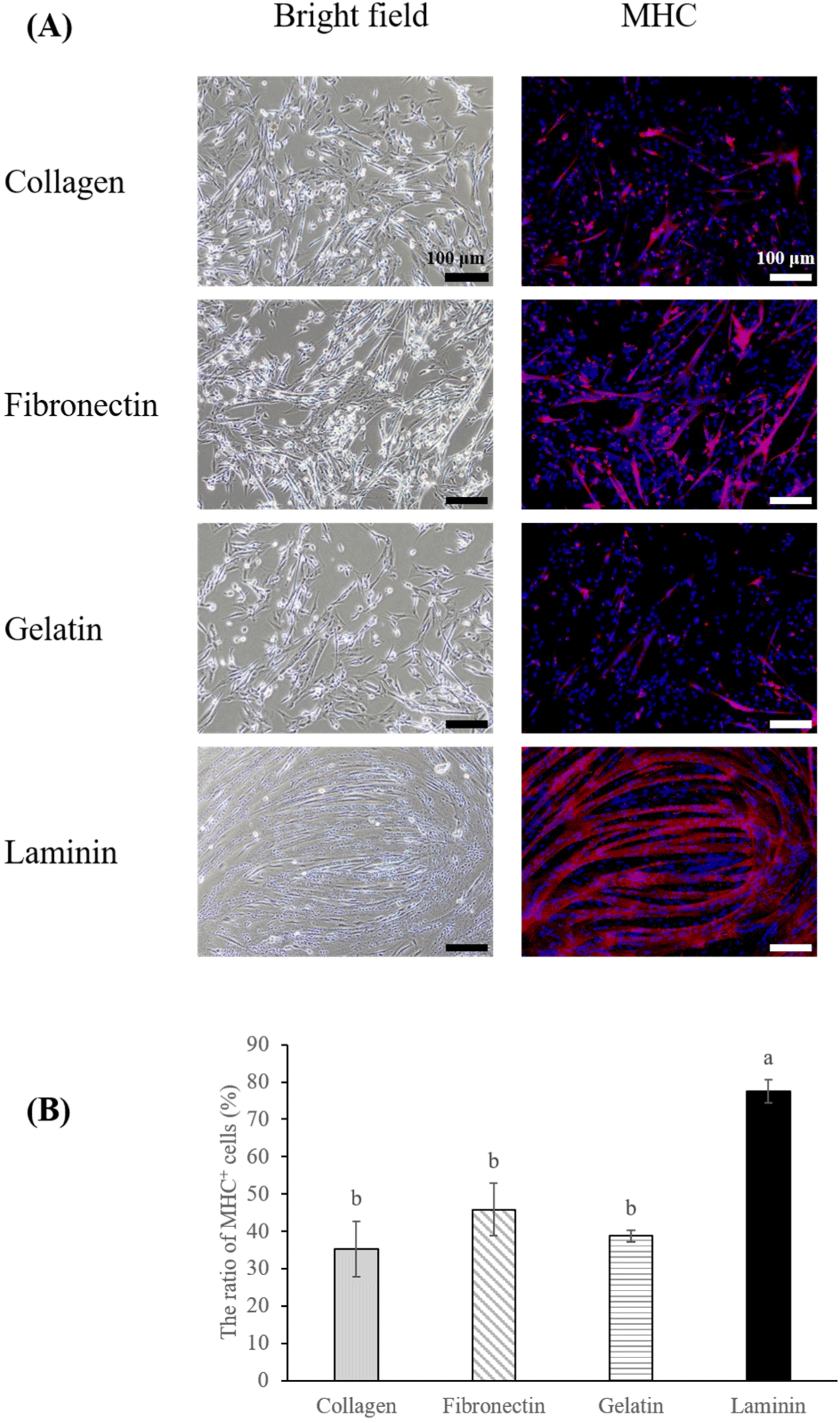
The differentiation potential of MuSCs is pivotal for accurately emulating real muscle tissue. Typically, serum starvation is employed to induce differentiation in MuSCs (Messmer et al., 2022), with the choice of coating materials also playing a role in the differentiation efficiency (Takahashi et al., 2022). In this study, we initiated the differentiation of MuSCs cultured on four different coating materials by implementing serum starvation on the third day of proliferation, at which cells had reached approximately 80%–100% confluency (Fig. 1A). Among the treatments, cells on laminin-coated dishes exhibited a higher differentiation ratio (Figs. 3A and B, p<0.05), and the differentiated muscle fibers on laminin were thicker and larger than those on collagen, gelatin, and fibronectin. In studies of C2C12 cells, cultures on fibronectin displayed more directional migration, alignment, and subsequent differentiation compared to collagen I or gelatin coatings (Chaturvedi et al., 2015; Vaz et al., 2012). However, our findings indicated that porcine MuSCs on fibronectin did not exhibit enhanced migration or differentiation compared to other coatings, contrasting with the results for laminin, which not only aligned by day three of proliferation (Fig. 1A) but also demonstrated superior differentiation. Furthermore, the observed decrease in MYF5 and MYOD in early muscle fibers (Bentzinger et al., 2013) partially supports our findings. We noted that MuSCs on laminin initiated differentiation more rapidly than those on other coatings, displaying cell alignment and lower expression of MYF5 and MYOD compared to those on gelatin by the third day of proliferation. Conversely, such differences in expression were not observed for MuSCs on collagen and fibronectin (Fig. 1B). Other studies indicated that human and mouse MuSCs cultured on Laminin 521 exhibited greater differentiation than those on gelatin or other laminin types (Penton et al., 2016), and bovine MuSCs on iMatrix-511 (laminin) showed higher differentiation than those cultured on gelatin or non-coated dish (Takahashi et al., 2022). This suggests the need for future research into how different types of laminin affect the differentiation of porcine MuSCs.
To further elucidate the differentiation into specific muscle fiber types based on mRNA expression levels, qPCR analysis was performed. The analysis focused on MYOG and muscle fiber isotype genes MYH1, MYH2, MYH4, and MYH7, which correspond to type IIx, type IIa, type IIb, and type I fibers, respectively (Eggers et al., 2021; Fig. 4). MuSCs cultured on laminin-coated dishes exhibited higher expression levels of genes associated with type IIx (MYH1) and type IIb (MYH4) fibers and showed lower MYOG expression compared to cells in other conditions (Fig. 4, p<0.05). MYOG, a crucial transcription factor in the myogenic regulatory pathway, is essential for differentiating myoblasts into matured muscle fibers. Its role is critical for both embryonic muscle development and the regeneration of adult skeletal muscle, facilitating the transition of MuSCs from proliferation to differentiation (Zammit, 2017). Studies on human and mouse myoblasts have shown that during the initial stages of differentiation, MuSCs express MYOG, which then decreases after myotube formation (Brown et al., 2012; Stern-Straeter et al., 2011). This suggests that MuSCs on laminin are differentiated much better than MuSCs on collagen, fibronectin, and gelatin, with increased myotube formation, consistent with Fig. 3A.
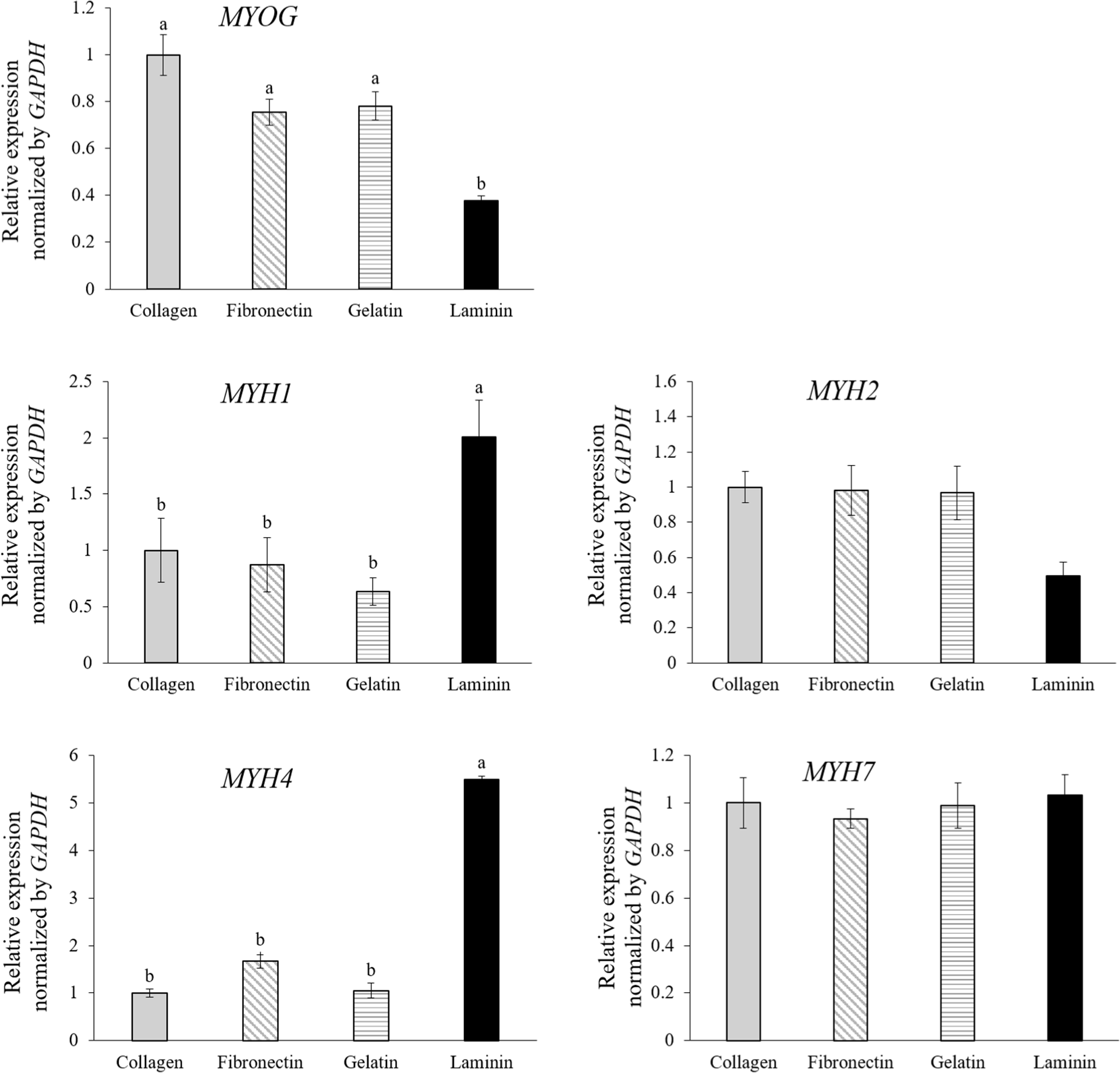
Force generation in skeletal muscles is achieved through the interaction between myosin and actin (Zammit, 2017). Muscle fibers are classified into slow oxidative type I or fast-twitch types IIa, IIx, or IIb using MYH isoforms (Zammit, 2017). All analyzed MYH isotypes in this study are adult MHCs, and an increase in the expression of these adult isotypes could indicate muscle fiber maturation (Chaturvedi et al., 2015). In the developmental studies, the primary and secondary myogenesis is distinguished (Chal and Pourquié, 2017), where MYH7-expressing fibers form during primary myogenesis, and MYH1, MYH2, and MYH4-expressing myofibers form during secondary myogenesis. Thus, muscle fibers on laminin-coated dishes may be more mature than others, aligning with a progression in muscle fiber expression from type I to type IIb during regenerative and developmental stages in fetal tissue. Moreover, the uniform expression of type I (MYH7) across all groups underlines the observation that all MuSCs underwent initial differentiation into early-stage muscle fibers, whereas those on laminin advanced to more mature fiber types. Numerous studies have demonstrated a close relationship between the type of muscle fiber and the quality of meat (Lee et al., 2023). Therefore, using a proper ECM coating like laminin could be a clue for the more maturated muscle fiber production in vitro, reflecting a closer similarity to real meat and better quality in cultured meat.
The enhanced differentiation observed might be related to myostatin inhibition, a known negative regulator of muscle growth and differentiation. Miura et al. (2010) and Yasaka et al. (2013) reported that laminin can bind to myostatin, attenuating its signaling. However, it is interesting to note that Miura et al. (2010) also found that fibronectin, which was not an effective coating material for porcine MuSCs in our study, can also bind to myostatin. This suggests that the relationship between myostatin binding and the efficacy of coating materials in promoting muscle differentiation is not straightforward. Further studies are essential to elucidate the specific interactions between laminin and porcine MuSCs and to analyze muscle maturation processes comprehensively.
Conclusion
This research highlights the limitations in 2D culture methods and short differentiation periods, pointing towards the necessity of employing 3D experimental setups for future work. Such endeavors could include comparing the effects of different ECM coatings on microcarriers or investigating the impact of various coatings on scaffolds designed for differentiation, offering a better understanding of muscle tissue engineering.
This study underscores laminin’s superiority over other ECM coating materials like collagen, fibronectin, and gelatin in promoting porcine MuSCs proliferation and differentiation, and even maturation, establishing it as a key coating material for muscle tissue engineering. Despite challenges related to its cost and sourcing, laminin’s effectiveness in mimicking matured muscle tissue presents a compelling case for its potential in cultured meat production and beyond. These challenges could be addressed through the use of recombinant protein technologies and microbial production, allowing for mass cultivation and potentially overcoming the economic barriers associated with laminin’s commercial application. Looking forward, the integration of laminin or its derivatives into 3D scaffolds emerges as a critical area for research, promising to enhance muscle cell differentiation further. This approach not only aligns with the goals of cultured meat production but also opens new avenues for in vitro muscle cultivation, setting a strategic direction for the field and highlighting laminin’s central role in advancing muscle tissue engineering.













