Introduction
Dry-cured ham, which has a long history of manufacturing and consumption especially in the Mediterranean and Asian regions, is the one of popular meat products worldwide (Chen et al., 2022; Jiménez-Colmenero et al., 2010; Zhou and Zhao, 2012). Dry-cured ham is highly preferred due to its unique taste and flavor (Estévez et al., 2008; Gaspar et al., 2022). The consumption of dry-cured hams per capita has been reported to be approximately 2.04 kg in some countries such as Spain (Chen et al., 2022).
In dried foods, the growth of bacteria causing spoilage and pathogenic are inhibited by low moisture content and water activity (Chitrakar et al., 2019). During the drying period, water removal, which induces cell shrinkage and damage, is the main mechanism responsible this phenomenon (Abee and Wouters, 1999). However, the components, such as amino acids and peptides, in dry foods are reported to have a possibility to improve the survivability of microorganisms (Morgan et al., 2006). Dry-cured ham, with its unique processing technique (dry salting and curing for a long period), is considered to be a shelf-stable meat product (Austrich-Comas et al., 2023). However, some studies have reported the presence of pathogens in dry-cured ham slices (Márta et al., 2011; Ng et al., 1997). Additionally, it has recently been noted that drying at low temperatures alone is inadequate in regard to reducing spoilage by microorganisms, which causing odor development in dry meat products (Chitrakar et al., 2019).
The definition of shelf life in meat products can be said the amount of time that passes before it becomes unacceptable in terms of appearance (discoloration) and human consumption. Meat products are composed of high nutrients, such as proteins and lipids, which are highly vulnerable to spoilage, causing quality deterioration. The organic amines and secondary products, which cause not only quality deterioration such as discoloration, off-flavor, and shortened shelf life of meat products, but also health concerns, are formed through the growth of spoilage bacteria, protein degradation, and lipid oxidation (Bekhit et al., 2021).
A combination of processing technologies and proper packaging systems is necessary for the minimization of quality deterioration and improvement of the shelf-life of meat products. Various packaging systems have been developed and applied in the meat industry.
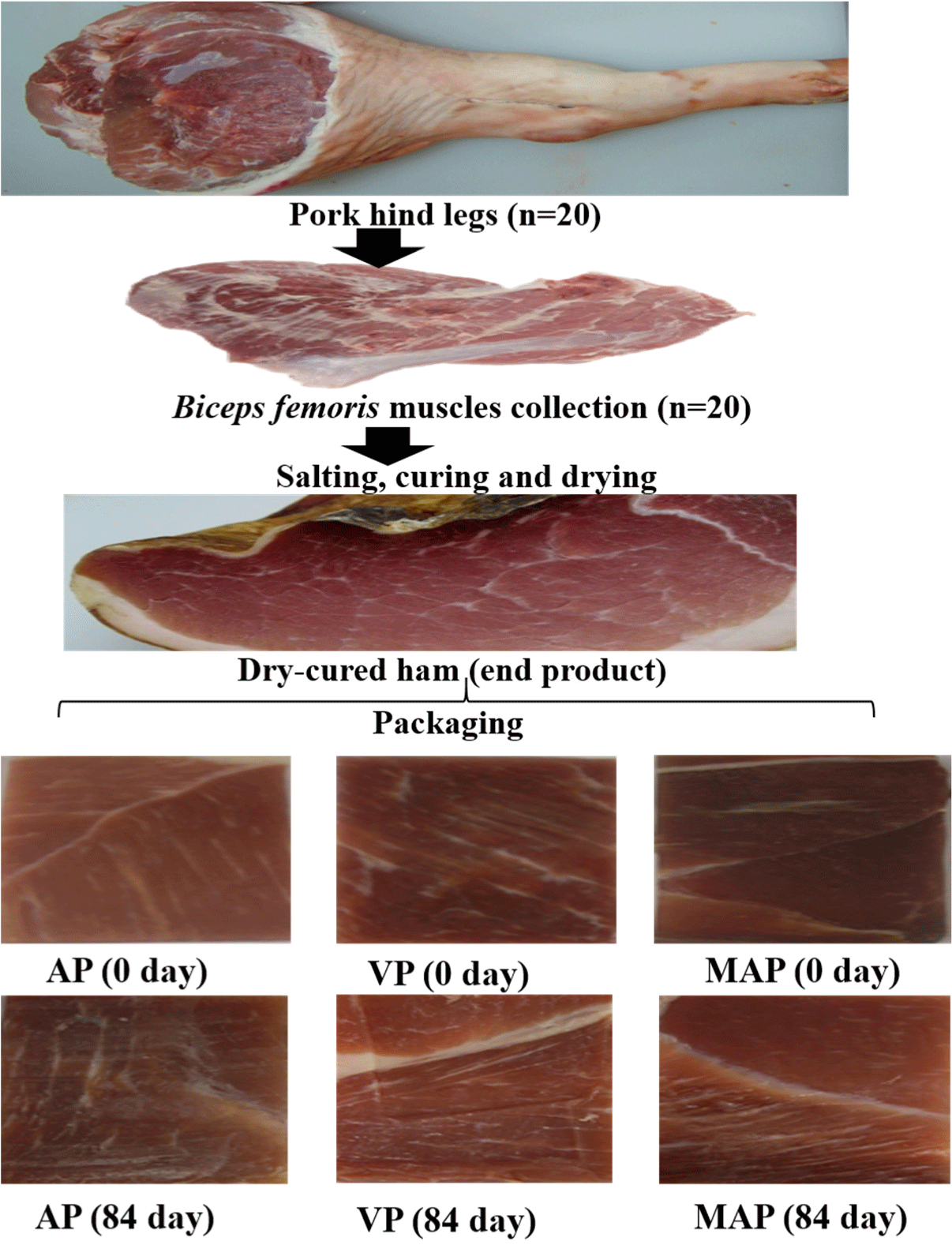
Dry-cured hams are typically processed from whole pig thighs or cuts. The final products are sold and distributed as whole pieces or cuts. In recent years, owing to changes in lifestyle, purchasing habits, and convenience, there has been an increasing demand for smaller sliced packed formats of dry-cured hams (León et al., 2023). However, to retain the eating quality, dry-cured ham slices must be packed using the proper packaging method. Only a few studies have focused on the determination of the shelf life in sliced, packaged dry-cured ham. Thus, the main objective of this study was to assess the effects of different packaging systems (aerobic, vacuum, and modified atmospheric packaging) on the shelf life stability of ready-to-eat dry-cured ham slices during storage.
Materials and Methods
A total of 20 hind legs (weight range of 12–14 kg) of commercial finishing pigs (Landrace×Yorkshire)×Duroc (LYD) at 24 h post-mortem was collected from a commercial abattoir (Jeonju, Korea) and used. The thighs were then dissected and only the biceps femoris (BF) muscles (average weight: 2.9 kg) were used for dry-cured ham processing. Salting was conducted by rigorously rubbing a salt mixture containing 5% NaCl, 0.01% NaNO2 and 0.05% sodium erythorbate onto the entire surface area of the BF muscle. Thereafter, a mixture of a starter culture containing Lactobacillus pentosus [4.0×109 colony forming unit (CFU)/g] and Staphylococcus carnosus (6.0×109 CFU/g) was applied on each sample surface. After salting, all the samples were placed on stainless steel shelves at 0°C–4°C and relative humidity of 60%–70% for 35 d. Following washing with cold water to remove excessive salts and drying with paper towels, the hams were left at 2°C–5°C and relative humidity of 70%–80% for 42 d. The ripening and drying process was conducted by hanging the hams on the stainless steel standing racks at 16°C and relative humidity of 70% for 12 mon. The hams were targeted at a weight loss of approximately 35% (compared their initial weight).
Upon completion of processing, the end products were sliced into ready- to-eat 2 mm-thick slices, placed into trays or bags (approximately 200 g each), and then subjected to one of the three packaging treatment groups. (i) aerobic packaging (AP): the slices were placed on trays and overwrapped with polyvinyl chloride film (oxygen transmission rate of 25,560 cm3/m2/24 h), (ii) vacuum packaging (VP): the slices were placed in polyethylene bags (oxygen transmission rate of 50 cm3/m2/24 h) and vacuum packed with a packager, and (iii) modified atmosphere packaging (MAP): the slices were placed on trays, introduced into bags, and replaced with 70% N2 and 30% CO2 gas mixture and then sealed with nylon film (oxygen permeability of 22.5 cc/m2/24h (Sunkyung, Seoul, Korea) using a Multivac T200 packaging machine (Haggenmüller, Enger, Germany). Twenty trays (bags) were prepared for each treatment group. The trays were stored in the 10°C cold room for 0, 18, 56, or 84 d, and 5 trays from each treatment was randomly selected and used for measurements.
The microbiological quality of products was assessed during storage. Briefly, immediately after opening the sample bags or trays, 10 g of samples were weighed and mixed with 90 mL of saline for 1 min using a Stomacher. Following a serial dilution with saline, the samples (1 mL each) was spread on petrifilm (3M Health Care, St. Paul, MN, USA) or petrifilm lactic acid bacteria (LAB) for the enumeration of total aerobic plate count (TAC) and LAB. The results were expressed as Log CFU/g.
Changes in the color of the products during storage were assessed using a color meter (Model CR-300, Minolta, Osaka, Japan). The calibration using a standard white tile (Y=93.50, x=0.3136, y=0.3198) was performed prior to use. After the tray or bag samples were opened, the color was measured directly at three different locations on the surface of each sample. The CIE L*, CIE b*, and CIE a* color traits were measured.
Three grams of samples from each treatment were weighed in 50 mL tubes and homogenized with 27 mL of distilled water at 14,000 rpm using a homogenizer. Then, the pH was determined using a pH meter (Sentron Argus-X, Roden, The Netherlands). Each sample was measured in triplicate.
The degree of protein degradation during the storage was determined by measuring total volatile basic nitrogen (TVBN) contents as described by Seong et al. (2014). Briefly, duplicate aliquots (5 g) of each sample were homogenized using a homogenizer (Polytron MR-2100, Kinematica AG, Malters, Switzerland) in a 100 mL tube containing 45 mL of distilled water. After filtering through a Whatman filter paper (No.1, AEC Scientific, Seoul, Korea), the resultant filtrates were collected and used for TVBN analysis. First, to minimize the leakage of volatile basic nitrogen from the samples, the Conway grass dishes were greased with Vaseline at their edges. Next, one milliliter of the sample and Conway’s reagent (0.066% methyl red: 0.066% bromocresol green, 1:1) were placed in the outer and inner spaces of the dish, respectively. Following the addition of 1 mL of 50% (w/v) K2CO3 to the outer space, the Conway dishes were immediately sealed with a cover and fixed with a metal clip. The samples were left at 37°C for 2 h and then various volumes of 0.01 N H2SO4 solution were applied onto the inner space until color changed to violet. Finally, TVBN content (mg/100 g) was calculated using the following formula:
where a is the volume (mL) of added H2SO4 to the sample, b is the volume (mL) of added H2SO4 to the blank, S is the weight (g) of the sample, and F is the factor of H2SO4.
Thiobarbituric acid reactive substance (TBARS) content of dry-cured ham slices packed with the different systems was measured by the modified method of Pikul et al. (1989) to determine the extent of lipid oxidation. Briefly, 5 g of samples were homogenized in 19.5 mL of 4% perchloric acid with addition of 0.5 mL of 7.5 butylated hydroxansole for 20 s at 13,000 rpm. After the filtering with Whatman filter paper, 5 mL of filtrate was mixed with 5 mL of 0.02 M thiobarbituric acid solution in 50 mL tube. Then the mixtures were heated at 80°C for 1 h in a water-bath. The absorbance of the samples was measured at 531 nm using a spectrophotometer (ProteomeLab Du-800, Beckman Coulter, Brea, CA, USA). The TBARS content (expressed as mg malondialdehyde/kg MA/kg) of the sample was calculated by multiplying the absorbance by a constant coefficient of 5.5.
A food scanner (model: 78810, Foss Tecator, Hillerød, Denmark) was used to determine the fat, protein, moisture and ash contents of the products. For this analysis, the samples (approximately 50 g each) were ground, placed in a Petri dish, and placed in an oven. Fat, protein, and moisture contents were determined and then expressed as relative percentages. Each sample was analyzed in duplicate.
The water activity (aw) value of the product was determined by the water activity meter (Novasina AW SPRINT-TH 300, Novasina, Pfäffikon, Switzerland). Salt content (%) of the samples was determined using a salinity meter (TM 30D, Takemura Electric Works, Tokyo, Japan).
The fatty acids (FAs) in the products were determined by the method described by Morrison and Smith (1964). Fifty grams of samples were homogenized in 150 mL of solvent mixture of methanol: chloroform (1:2 ratio) with 1.0 mL of internal FA standard (C13:0, 0.5 mg/mL in methanol) for 3 min at 2,500 rpm. The samples were then filtered using Whatman filter paper, and the resultant filtrates were added to a 250 mL flask containing 20 g Na2SO4. The samples were then concentrated at 55°C using a rotary evaporator. The resultant concentrated sample was placed into a test tube containing 1 mL 0.5N-NaOH (2 g NaOH/100 mL methanol) and heated at 100°C for 20 min. After cooling, 2 mL BF3-methanol was added to each the sample tube and again heated at 100°C for 20 min. After cooling for 30 min, 1 mL heptane and 8 mL NaCl were added to the sample tube and mixed for 1 min. The FA samples were analyzed using a capillary column (30 m×0.32 mm i.d×0.25 μm film thickness) connected with a gas chromatography/flame ionization detector (8890 model, Agilent Technologies, Santa Clara, CA, USA). The oven initial temperature was 140°C and raised to 230°C at a rate of 2°C/min. The injection and detector temperatures were set at 250°C and 260°C, respectively. Nitrogen was used as the carrier gas. FAs were identified using standard FAs (FAME Mix CRM47885, Sigma-Aldrich, St. Louis, MO, USA). Individual FAs are expressed as relative percentages (%) of total FAs.
The free amino acid (FAA) content of the products was determined using the procedure described by Cho et al. (2020). Samples (5 g each) were placed in a 50 mL test tube and homogenized with 10 mL distilled water at 11,000 rpm for 1 min. Following centrifuging at 20,784×g at 4°C for 10 min, the supernatant (100 μL) was carefully taken and mixed with 900 μL methanol containing 0.1% formic acid. The mixture was again centrifuged at 20,784×g at 4°C for 10 min and the supernatant was transferred to 0.2 mL vials for FAA analysis. The samples (5 μL each) was injected into a high performance liquid chromatography (Dionex Ultimate 3000 model, Thermo Fisher Scientific, Waltham, MA, USA). Two solvents [acetonitrile (100 mM ammonium formate, 20:80 v/v] and solvent B (acetonitrile: trifluoroacetic acid: 25 mM ammonium formate: formic acid: 9:75:16:03 v/v/v), were used to move the FAAs. The column elution speed was set at 0.5, with 100% solvent B (0–3 min), 83% B (4–6.5 min), 0% B (6.6–10 min), and 100% B (11–17 min). The detection was monitored at 260 nm. Amino acid standard mixtures (Sigma-Aldrich) at different concentrations (10, 20, 50, and 100 nmol/mL) were separated under the same conditions and used for identification and quantification of FAAs. The results were expressed as milligrams per 100 g of meat (mg/100 g meat). On the other hand, to examine whether the packaging and storage alter the tastes of the products, the FAAs were grouped (based on their similar taste quality) into: sweet amino acids (SAA: threonine, serine, glycine, alanine), aromatic amino acids (AAA: tyrosine, tryptophan and phenylalanine) and bitter amino acids (BAA: valine, methionine, isoleucine, tyrosine, phenylalanine, histidine and arginine; Dashdorj et al., 2013; Kato et al., 1989; Sforza et al., 2006).
A two-way analysis of variance (ANOVA) was used to analyze the data. In the General Linear Model procedure of SAS (SAS Institute, Cary, NC, USA; 2007), packaging and storage were set as the main effects and the obtained results were set as dependent variables. Duncan’s Multiple Range Test was adopted to compare the mean differences. Statistical significance was set at p<0.05. significant. Data are presented as means±SD.
Results and Discussion
As shown in Table 1, the mean values of moisture, protein, and fat of the products among the treatments measured after 0 d storage ranged from 45.84±0.99% to 46.78±0.58%, 21.15±0.81% to 21.62±0.53%, and 22.96±0.93% to 23.74±0.23%, respectively. No differences in these contents were observed among the various treatments (p>0.05). The water activity (aw) value of the products ranged from 0.85–0.86. For dry food products, water activity is a very important parameter that indicates the remaining moisture content or degree of dryness, which significantly affects the deterioration rate and shelf-life stability of the products (Erkmen and Bozoglu, 2016). The moisture content and aw value of the products in our study were similar to those reported for Iberian, Norwegian, and Korean dry-cured hams (Carrapiso and García, 2008; Jin et al., 2012; Petrova et al., 2016) but lower than those of Italian dry-cured ham products (Laureati et al., 2014).
Salt content is a very important factor that contributes to the typical taste and shelf-life stability of dry-cured ham products (Ruiz-Ramírez et al., 2006). In our study, all the products contained a salt level of 6.67%–7.33%, which was lower than those reported in Norwegian and Italian dry-cured hams (Laureati et al., 2014; Petrova et al., 2016), and higher than the level reported in Iberian dry-cured ham (Carrapiso and García, 2008). These contrasting results are attributed to the differences in manufacturing conditions (e.g., duration and temperature used for drying products) among the studies.
TAC and LAB are important indicators of microbiological quality during processing, distribution, and storage of meat and meat products (Baer et al., 2013). As shown in Table 2, for all the samples had an initial TAC and LAB counts (0 d) of 6.84–7.35 Log CFU/g and 5.10–5.42 Log CFU/g, respectively. The similar results were found in Piras et al. (2016) for vacuum-packed dry-cured ham slices, but different with Kim et al. (2014) which reported higher results in vacuum-packed dry-cured pork shoulder slices. After 84 d of storage, TAC increased by 0.84, 1.15 and 1.24 Log CFU/g in AP, VP, and MAP, respectively. While, the total LAB increased by 0.63, 1.24 and 1.12 Log CFU/g in the AP, VP and MAP, respectively. In general, all samples exhibited high microbiological stability during storage. Therefore, no inhibitory effects against spoilage bacterial growth were observed in these packaging systems. This was probably due to the inherent environment of the product (e.g., high salt and low moisture), which was unfavorable for growth. Similar to our results, Piras et al. (2016) reported a slow increasing rate of TAC in dry-cured ham slices with vacuum package after 63 d of storage at 25°C. Rubio et al. (2007) reported high microbiological stability of dry-cured beef products under VP conditions during storage.
TVBN is commonly considered as a biomarker of protein degradation in meat products during processing, distribution, and storage. TVBN formation is closely associated with various spoilage mechanisms (microbial growth and autolysis by endogenous enzymes), which may result in quality deterioration of meat products (Bekhit et al., 2021). We observed that the TVBN concentration was significantly higher in the AP-packed samples than in the VP- and MAP-packed samples over the storage period (p<0.05; Table 3). A comparison of the TVBN content between VP and MAP showed a higher (p<0.05) level in samples packed with VP after 84 d of storage. With respect to the effect of storage, the TVBN in the AP-packed samples increased significantly (p<0.05) after 56 d and did not increase thereafter (increased by 25 mg after 84 d of storage). The weight of the VP-packed samples did not increase within 28 d but increased by 8.63 mg and 28 mg after 56 and 84 d of storage, respectively. While, the MAP samples showed the lowest TVBN increasing rate (by 7.9 mg and 19 mg, after 56 and 84 d, respectively). This indicates that the rate of TVBN formation in the samples was largely affected by the packaging system, in which MAP exhibited a better preservative effect. Cilla et al. (2006) reported a significant increase of TVBN in VP or MAP-packed dry-cured ham slices during refrigerated storage at 4°C, which was in agreement with our results. However, compared to the TVBN content (75–96 mg/100 g) reported by these authors, our results showed lower levels. Similarly, Lee and Kim (2023) reported an increase in TVBN in dry-cured beef with an increase in manufacturing and storage time. As mentioned above, the TVBN formed in meat and meat products results from spoilage mechanisms such as microbial growth or autolysis. In our study, the total spoilage bacterial count did not differ among the treatments. Therefore, the different rates of protein degradation by endogenous enzymes could be the mechanism responsible for the TVBN results.
At the initial measurement (0 d), the range of pH values from each treatment were from 5.14 to 5.22, which showed numerical differences (p>0.05). However, the VP- and MAP-packed samples had lower pH level compared to the AP at the end of storage (84 d; p<0.05). This result can be resulted by the higher total LAB counts in these samples (Table 2). The pH of samples packed with AP and VP increased, whereas that of samples packed with MAP decreased, with increasing storage time (p<0.05). Supporting these findings, Cilla et al. (2006) reported a similar trend in pH evolution in dry-cured ham packed with MAP (20% CO+80% N2) during storage. The pH is generally considered an important factor affecting the shelf-life stability of fermented meat products. Compared with the pH values (6.2–6.5) reported for dry-cured ham products manufactured without starter culture inoculation (Cilla et al., 2006; Kurek et al., 2021; Seong et al., 2014) all our samples showed considerably lower values. In recent decades, starter cultures have been widely used to fortify the shelf life of fermented foods by enhancing acidic environments. Jin et al. (2012) and Kim and Kim (2023) used starter cultures and reported a low pH (approximately 5.0) for dry-cured ham products.
The oxidation of lipids, particularly unsaturated FAs (UFAs), as a result of the free radical mechanism, is the major cause of off-flavor development, discoloration, and quality deterioration of meat products (Domínguez et al., 2019). Over the storage period, the TBARS content was significantly higher in the AP-packed samples than in the VP- and MAP-packed samples (p<0.05; Table 3). However, no significant differences were found in the TBARS content between the VP and MAP treatments regardless of storage days (p>0.05), indicating that both VP and MAP showed a similar protective effect against lipid oxidation. With an increasing storage time, the TBARS values of dry-cured ham slices from the three different packaging methods were significantly increased (p<0.05). As expected, samples packed with VP and MAP showed lower rates of lipid oxidation than those packed with AP. Particularly, after 84 d of storage the TBARS value increased by 1.44, 0.58 and 0.67 mg MA/kg in the AP, VP and MAP, respectively. A similar phenomenon was also observed by Kurek et al. (2021) for dry-cured ham packed with VP or MAP during storage at 4°C. Compared with the TBARS levels (2–6 mg MA/kg) of dry-cured pork shoulder slices packed with VP or MAP at 90 d of storage (Kim et al., 2014), our samples showed much lower levels. Regarding the TBARS results observed in the present study, it may be explained that the presence of oxygen could be the main cause resulting in the accelerated lipid oxidation in the AP-packed samples because oxygen is known to be the most important reactant in this reaction process (Domínguez et al., 2019; Johnson and Decker, 2015). In contrast, VP and MAP were more effective at retarding lipid oxidation in dry-cured ham slices over the storage period.
Color is well known factor that determining the quality of meat and meat products. During storage, lipid oxidation is the major mechanism contributing to the discoloration of meat products (Papuc et al., 2017). Furthermore, alteration of the structure of protein pigments during storage significantly affects their light-scattering capacity, which consequently affects the color of meat products (Guo et al., 2021). As shown in Table 4, the initial CIE L* were higher in the AP-packed samples (44.55) than in the VP-(35.51) and MAP-packed samples (32.13; p<0.05). However, at the end of storage (d 84), the CIE L* were similar in all the three AP, VP and MAP treatments (p>0.05). With regard to CIE a*, the AP-packed samples had a similar value to VP, and a higher value than MAP at 0 d. However, after 84 d of storage, the CIE a* decreased to 8.51 (VP), 7.87 (MAP), and 6.16 (AP), showing significant differences between the treatments, respectively (p<0.05). The increasing trend of CIE L* and decreasing trend of CIE a* during storage were found in the present study. Increased CIE L* values of dry-cured meat during storage have been attributed to the formation of cloaks or white films on the meat surface (Rubio et al., 2007). Similar observations have been reported by Guo et al. (2021), Kim et al. (2014), and Piras et al. (2016) for dry-cured ham and dry-cured mutton ham packed under vacuum during storage. CIE a* is considered the most important attribute in regard to attractiveness by consumers. The results of instrumental color measurements show the considerable effect of the packaging system on dry-cured ham slices, and the MAP system had a higher protective effect on discoloration during storage compared to AP and VP. The AP-packed samples were almost completely discolored on d 84 of storage (Fig. 1), probably due to accelerated lipid and protein oxidation as a result of the presence of oxygen in the packaging headspace (Table 3). Consistent with our results, Cilla et al. (2006) reported a slightly higher CIE a* value in dry-cured ham slices packed under MAP than those packed under VP. Rubio et al. (2007) also reported higher CIE a* stability in dry-cured beef products packed under MAP conditions than those packed under AP after 210 d of storage.
Taste is the one of the major factors that determining the eating quality and satisfaction of meat products. The taste of meat products is mainly generated by non-volatile molecules, such as FAAs (Khan et al., 2015; Mateo et al., 1996; Sforza et al., 2006). At the initial measurement, no difference in the total sweet, aromatic, and bitter FAAs contents was observed among the various treatments (p>0.05). On 28 d and 56 d, the VP-packed samples contained a higher sweet FAAs content than the AP- and MAP-packed samples (p<0.05). However, as the storage time was prolonged to 84 d, the samples in all packaging systems showed similar sweet FAAs levels (p>0.05). A similar trend was observed for aromatic FAAs content in all of the treatments during storage. The significantly higher contents of total bitterness-related FAA were found in the AP system than in the VP and MAP system after 56 and 84 d of storage (p<0.05) Some previous studies have assessed the effects of packaging systems on the quality of dry-cured ham products during storage as above-cited (Table 5; Cilla et al., 2006; Piras et al., 2016; Rubio et al., 2007). However, changes in taste-related FAAs content in the products during storage were not measured in these studies. In our study, a decreasing trend in total sweet, aroma, and bitter FAAs content was observed in all samples with increasing storage time. Particularly, the total sweet FAAs content was reduced by 0.45, 0.28 and 0.31 mg/100 g in the AP, VP and MAP-packed samples after 84 d of storage, respectively. The total aromatic FAAs content also was reduced by 0.27, 0.18 and 0.19 mg/100 g in the AP, VP and MAP-packed samples after 84 d of storage, respectively. While, the total bitter FAAs content was reduced by 0.58, 0.30 and 0.39 mg/100 g in the AP, VP and MAP-packed samples after 84 d of storage, respectively. These results suggest that the taste intensity of the sliced dry-cured hams tends to decrease with increased storage time, regardless of packaging treatment. Researchers have stated that in cured meat products, a large number of FAAs and their derivatives are generated as a result of extensive proteolysis by endogenous and microbial enzymes, which contribute to the typical flavor of these products (Toldrá, 2006). In the present study, the decrease in taste-related FAAs in the product during storage could be related to the increased enzymatic deamidation of ammonia and/or decarboxylation of biogenic amines (Alfaia et al., 2004). A previous study also found a significant change of FAAs content (e.g., a decreased amount of SAA) in dry-cured ham during the ripening period (Salazar et al., 2020; Sforza et al., 2006). Based on these results, it may be said that the use of VP and MAP could partly reduce the loss of taste-related FAAs in sliced dry-cured ham during storage when compared to the AP system.
FAs are recognized as important components because they reflect the nutritional value and they also contribute to the organoleptic characteristics (e.g., odor intensity) of meat products (Salazar et al. 2014). There is no study that evaluated changes in FAs in dry-cured ham products with different packaging systems during the storage. In the present study, the FA composition of dry-cured ham slices packed with AP, VP, and MAP was assessed at the beginning (0 d) and end (84 d) of storage. Our results (Table 6) show that eight FAs, including three saturated FAs (C14:0, C16:0, and C18:0) and five UFAs, were detected in all of the samples studied. The levels of all UFAs, including C16:1n7, C18:1n9, C18:2n6, C18:3n3, and C20:4n6, decreased significantly after 84 d of storage (p<0.05). We found that PUFAs (e.g., C18:2n6, C18:3n3, and C20:4n6) showed a larger decrease than monounsaturated fatty acids (MUFAs; e.g., C16:1n7, C18:1n9). After 84 d of storage, the C18:3n3 content decreased by 50%, 48%, and 42%, and the total UFAs content decreased by 9.02%, 5.13%, and 6.11% (compared with the initial level) in the AP, VP, and MAP-packed samples, respectively. During processing (salting, curing, and ripening), lipolysis activity taking place is the main mechanism responsible for the decreased UFAs content in dry-cured ham products (Salazar et al., 2016). Additionally, because of their unstable structures (more double bonds), PUFAs are easily oxidized by oxidizing agents (e.g., oxygen), leading to a decrease in PUFAs in meat products during processing (Domínguez et al., 2019). Regarding this, numerous studies have reported a considerable decrease of UFAs in dry-cured ham with prolonged manufacturing time (Salazar et al., 2020; Storrustløkken et al., 2015). Based on our results, either continuous lipolysis or accelerated lipid oxidation (Table 3) during storage could be the reason for the decreased UFAs seen in all samples. However, the use of VP and MAP exerted a better effect in retarding lipolysis and/or lipid oxidation activities, which increased the stability of PUFAs in the dry-cured ham slices during storage than AP.
Conclusion
In the present study, the quality and shelf life of ready-to-eat dry-cured ham slices packed under various packaging systems (aerobic, vacuum, and MAP) were investigated over 84 d of storage. There was a substantial change seen in the physicochemical properties of the products during storage, regardless of the packaging. The color, lipid and protein oxidation stability, taste-related amino acids, and FA content of the products are largely influenced by the packaging systems. When compared to AP (overwrapping), both vacuum and MAP systems exerted a higher protective effect against spoilage mechanisms during storage. However, MAP had a better effect on the retardation of protein and lipid oxidation and discoloration than AP. Under the AP condition, the products could retain its color stability up to 28 d of storage at 10°C. Overall, ready-to-eat dry-cured ham product packed under the vacuum or modified atmosphere (70% N2 and 30% CO2 gas mixture) is recommended to retain the quality, taste, and nutritional value during storage. Overwrapping is also useful in terms of the convenience of packaging, depending on the types of distribution (especially on the showcase for short distribution).













