Introduction
The human skeletal system contains 206 bones which play a role in support and movement as well as in protection of vital organs. Being a dynamic organ, bone continuously goes through remodeling process which consist of bone resorption and formation. Lack of balance in this process can lead to a variety of bone problems e.g. osteoporosis (Datta et al., 2008; Robling et al., 2006). Bone diseases encompass a wide range of conditions that affect the structure, strength, and function of bones. These diseases can be caused by genetic factors, nutritional deficiencies, infections, or other medical conditions (Singh et al., 2023).
Dairy products, especially fermented milk products and those which have low-fat content offer a variety of well-reported benefits. These products contain essential minerals like calcium, phosphorus, and vitamin B12 and vitamin D, all of which improve bone health (Saleem et al., 2024). Moreover, nutrients particularly calcium from dairy products is often considered more bioavailable than calcium from other alternatives (Geller et al., 2022). In addition to providing the body with critical nutrients like vitamins and minerals, dairy functional foods improve the gut epithelial barrier and modulate mucosal immune system (Illikoud et al., 2022). These also promote fat excretion and enhance metabolic health; calcium content can reduce blood pressure by inhibiting 1,25-dihydroxyvitamin D. Yogurt has beneficial impact on lipid profile, reduces cholesterol and regulates glucose control thereby lowering type 2 diabetes mellitus (Feng et al., 2022). In addition, fermented dairy products improve lipid metabolism and antioxidant status which may help improve cardiovascular health (Companys et al., 2020).
The term probiotics refers to the microorganisms that, when taken in appropriate amount, confer beneficial impacts on health (Hill et al., 2014). Lactobacillus and Bifidobacterium are the most well-known genera in this regard, however, Bacillus, Pediococcus and some yeasts species also possess probiotic properties. Probiotics have been described to have beneficial roles ranging from extending the shelf-life of the products to amelioration of specific intestinal and extra-intestinal disorders as well as cognitive improvement (Hasnain et al., 2024; Jang et al., 2024b; Lee et al., 2023).
Bone health is influenced by a variety of intrinsic and extrinsic factors, including genetics, nutrients and environmental factors. Among dietary determinants, dairy products are a significant contributor due to their rich content of bone-supportive nutrients and probiotics. Fermented dairy products ensure enhanced bioavailability of minerals, particularly calcium by lowering intestinal pH. In addition, probiotics’ short-chain fatty acids (SCFAs) also supports calcium absorption. Consumption of mineral-fortified dairy products reduces serum parathyroid hormone (PTH) levels and bone resorption markers (e.g., TRAP 5b and CTX), reducing bone loss. Regular dairy consumption improves bone mineral density (BMD), bone mineral content (BMC) and bone strength in children adolescents and in women particularly reducing osteoporosis risk. Moreover, these provide proteins, magnesium, phosphorus, and insulin-like growth factor I (IGF-I), thereby contributing bone remodeling and repair (García-Burgos et al., 2020).
This review aims to critically evaluate the existing literature on the role of various bone health determinants and highlights the significance of fermented dairy products in promoting bone health with a focus on their nutrient and probiotic content. The positive and negative factors for bone health are depicted in Fig. 1.
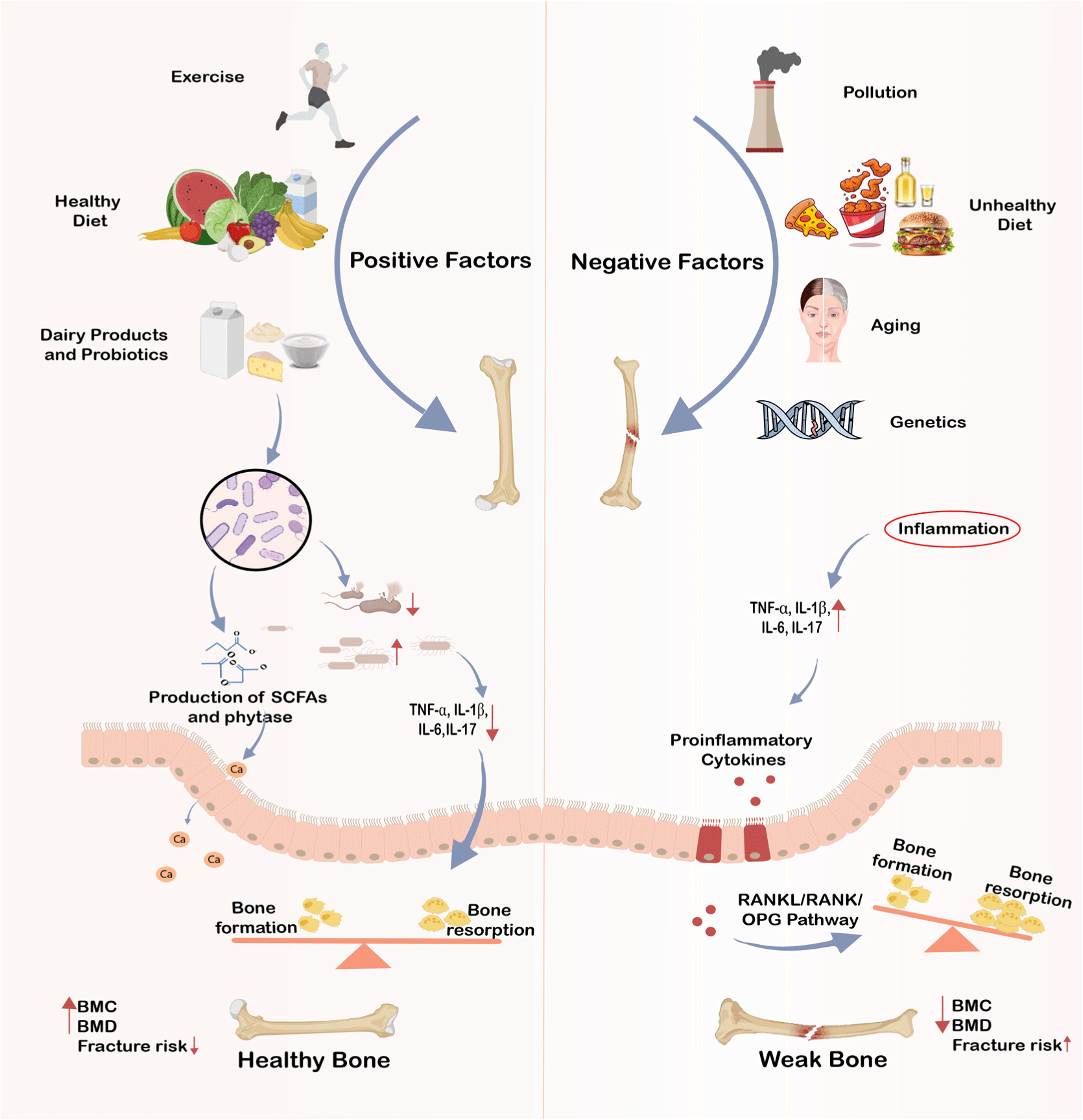
Bone as a Dynamic Organ
The bone is an important organ of the body, playing a role not only in support and movement but also in the protection of the soft tissues and vital organs. In addition, bone is critical for mineral storage, homeostasis and hematopoiesis, and serves some endocrine functions (Florencio-Silva et al., 2015; Fukumoto and Martin, 2009). Being a dynamic organ, bone is continuously in the process of remodeling mediated by the signals from bone cells and immune, hormonal and neural systems. It is estimated that around 5%–10% of the bone is replaced each year. This process is mediated by osteoblasts, the bone forming cells; osteoclasts, the cells responsible for bone degradation; osteocytes, which act as mechano-sensors and endocrine cells; and the bone-lining cells, which form bone canopy and couple bone formation and resorption, collectively termed as bone remodeling unit. The remodeling cycle has three main phases: bone resorption, transition, and bone formation (Florencio-Silva et al., 2015).
Probiotics favor bone remodeling through multiple mechanisms, primarily mediated by their interactions with the immune system and their production of beneficial metabolites. Key probiotic species, such as Lactobacillus spp. and Bifidobacterium spp., modulate the balance between pro-inflammatory Th17 cells and anti-inflammatory Treg cells. Th17 cells promote osteoclastogenesis by secreting interleukin (IL)-17 and RANKL, leading to bone resorption, while Treg cells inhibit osteoclast activity by producing anti-inflammatory cytokines such as IL-10 and TGF-β, enhancing osteoblast-driven bone formation. Additionally, probiotics enhance the production of SCFAs like butyrate, which suppress osteoclast differentiation and stimulate Treg cells to promote Wnt signaling and bone anabolism. Increase in serum osteocalcin, serum calcium levels, upregulation of Runx2 and Bmp2 genes, enhanced alkaline phosphatase activity and reduction in the serum CTX-1 levels, all of which favor bone formation and reduce bone resorption have been observed because of probiotics treatment. Probiotics also reinforce intestinal barrier integrity, reducing systemic inflammation and its negative impact on bone health (Amin et al., 2020; Bose and Sharan, 2024; de Sire et al., 2022; Lyu et al., 2023).
Bone Health Determinants
Genetic factors are the most significant contributors in bone health determination. BMD, a key determinant of bone health, is varied as high as 60%–90% due to genetic factors (Duncan et al., 2003; Recker and Deng, 2002). Bone health traits like BMD, height and strength are influenced by genes (Krall and Dawson‐Hughes, 1993). Some of the genes like LRP5 and SOST are critical for bone health and remodeling influencing overall bone strength and fracture susceptibility (Duncan and Brown, 2010). Several single nucleotide polymorphisms have been reported to influence an individual’s susceptibility to bone diseases (Mäkitie et al., 2019). Mutations in genes such as CLCN7, TCIRG1, and IKBKG are primarily responsible for various forms of osteopetrosis. These genes regulate the formation, growth, and function of osteoclasts, and mutations in one or more of them can lead to osteoclast dysfunction or loss (Stark and Savarirayan, 2009). Additionally, Paget’s disease of bone (PDB) is largely influenced by genetic factors, with mutations or polymorphisms identified in four key genes: TNFRSF11A, TNFRSF, VCP and SQSTM. All these genes are part of the RANK-NFκB signaling pathway, and their mutations enhance the risk of PDB by disrupting normal signaling eventually leading to osteoclast overactivation (Ralston, 2008). Ongoing research is focused on identifying specific genes that regulate bone mass and affect the risk of bone diseases (Ralston, 2008; Singh et al., 2023). Understanding the genetic basis of bone diseases may enable better screening, treatment, and prevention strategies for conditions like osteoporosis and age-related bone loss (Boskey and Coleman, 2010).
Aging influences the mechanical properties and composition of bones. Bone growth starts during fetal life and continues until the second decade of life; peak bone mass is reached at about 20 years of age which allows us to withstand mechanical overload (Rizzoli, 2014; Rizzoli and Biver, 2018). While growing old, the reduction in BMD leads to weaker and more brittle bones. Bone composition also shifts with growing age; collagen and other organic compound may degrade, and proportion of minerals increase making bones more brittle. It’s been reported that cortical bones become weaker and more brittle with age (Tommasini et al., 2007). Bone shape changes with age due to processes like cortical drift, where the outer diameter of bones expands, and the cortical walls become thin, leading to a reduction in bone strength. These changes contribute to bones becoming more fragile and prone to fractures (Boskey and Coleman, 2010).
Dietary patterns significantly impact bone health, with certain diets promoting higher BMD and reducing fracture risk, while others contribute to bone fragility (Movassagh Elham and Vatanparast, 2017). Beneficial diets, such as the Mediterranean diet which predominantly consists of fruits, vegetables, whole grains, healthy fats, and dairy-based patterns with low-fat and fermented dairy products, enhance calcium absorption, improve bone turnover biomarkers, and reduce bone resorption thereby improving bone health and reducing fracture risk (Benetou et al., 2013; Chen et al., 2016; Rivas et al., 2013; Zeng et al., 2014). High nutrient-density diets, rich in protein, calcium, and potassium, along with dietary diversity, also support stronger bones and lower osteoporosis risk. Conversely, harmful patterns like the Western diet, high in processed foods, soft drinks, fast food and red meat, as well as high-fat and low-nutrient diets, deplete essential nutrients and increase bone resorption, raising the risk of osteoporosis and fractures (Langsetmo et al., 2010; Langsetmo et al., 2016; Park et al., 2012; Zeng et al., 2013).
Environmental pollutants such as heavy metals, bisphenols, phthalates, and particulate matter can disrupt bone homeostasis by affecting hormonal balance, osteoblast function, and osteoclast activity. These toxins interfere with calcium metabolism by altering the regulation of hormones like calcitonin, PTH and vitamin D resulting in increased calcium release from bones and reduced absorption from dietary sources (Kheirouri et al., 2020; Prada et al., 2020; Zhang et al., 2020). Pollutants can also enhance osteoclast activity, reduce osteoblasts formation, enhance oxidative stress and systemic inflammation, accelerating bone resorption and inhibiting bone formation. Long-term exposure, even at low levels, may cause significant bone disorders, including osteoporosis, due to cumulative damage to bone cells and the extracellular matrix (Rodríguez and Mandalunis, 2018; Singh et al., 2023; Zhang et al., 2020).
Physical activity significantly enhances bone health by stimulating osteocytes through mechanical forces from muscle contractions and gravitational loading (Hong and Kim, 2018). Weight-bearing and resistance exercises, particularly high-impact activities like running and jumping, increase BMD, bone size, and strength, especially at weight-bearing sites like the hip and spine. These mechanical forces lead to adaptive changes in bone structure, promoting bone formation (Benedetti et al., 2018; Ishikawa et al., 2013). Physical inactivity, on the other hand, accelerates bone loss, while lifelong exercise helps preserve BMD and reduces fracture risk. Targeted resistance training also benefits non-weight-bearing sites, emphasizing that both muscle contraction and gravitational loading are crucial for maintaining bone health and preventing osteoporosis (Carter and Hinton, 2014).
Inflammation impairs bone health by tipping the balance towards bone resorption through inflammatory mediators like tumor necrosis factor-α (TNF-α), IL-6, IL-1, and prostaglandins (PGEs). These mediators alter the RANK/RANKL signaling pathway, stimulating osteoclast formation and activity (Singh et al., 2023). TNF-α induces RANKL production, while IL-1 and IL-6 increase PGE2 levels, promoting bone breakdown (Epsley et al., 2021). Mast cells and immune cells also release inflammatory signals, accelerating resorption. Bradykinin and neuropeptides like substance P contribute indirectly by affecting vascular permeability and prostaglandin synthesis (Dobigny and Saffar, 1997; Konttinen et al., 1996). Chronic inflammation in aging, or “inflammaging,” leads to oxidative stress and the buildup of advanced glycation end products, disrupting bone remodeling and reducing bone mass (Sanguineti et al., 2014). Despite known pathways, treatments targeting inflammation-induced bone loss are limited, highlighting the need for more research.
Bone Disorders and Potential of Dairy for Their Improvement
A fine balance between bone production and resorption keeps bones healthy, regulated by a complex interplay of hormonal, genetic, and environmental factors. Disruption in this balance can lead to a variety of skeletal disorders, each with unique underlying mechanisms that affect bone structure, strength, and function. Comprehending these pathways is essential to creating targeted treatments and improving patient outcomes. This section explores the pathophysiological processes of key bone-related diseases, shedding light on the molecular and cellular dynamics that drive their progression.
As a systemic skeletal disease, osteoporosis is characterized by decreased bone mass. An imbalance between bone resorption and production causes osteoporosis, where osteoclast activity surpasses that of osteoblasts, resulting in decreased bone mass and elevated fracture risk (Föger-Samwald et al., 2020). Estrogen deficiency, particularly post-menopause, is a key driver, as estrogen regulates osteoblast activity, suppresses osteoclast differentiation via the RANKL/OPG system, and lowers pro-inflammatory cytokines like IL-6 and TNF-α that would otherwise encourage bone resorption (Weitzmann, 2013). Estrogen loss also activates T-cells, especially Th17 cells, which secrete IL-17, further increasing osteoclastogenesis (Bhadricha et al., 2021). Osteoimmunology reveals that immune cells, such as memory T-cells and dendritic cells as well as proinflammatory cytokines, promote osteoclast activity through RANKL signaling, with chronic low-grade inflammation worsening bone loss (Yang and Zhu, 2024). Oxidative stress from reactive oxygen species hinders osteoblasts and promotes osteoclasts, and estrogen deficiency further reduces antioxidant enzymes like superoxide dismutase (Marcucci et al., 2023). Epigenetic changes, such as abnormal DNA methylation of osteogenic genes like RUNX2, suppress osteoblast differentiation, while hypomethylation increases osteoclast activity. Histone modifications and sirtuin 1 activity also influence bone cell function, with aging contributing to decreased bone formation. The gut microbiome affects bone health indirectly by influencing nutrient absorption and immune responses, and modulating osteoclast activity through the process of producing SCFAs (Xu et al., 2021). Fermented dairy and its probiotic content improve the BMD as well as BMC via several mechanisms e.g. i) enhanced supply and bioavailability of essential minerals ii) reduction in pro-inflammatory mediators, iii) modulation in pro-inflammatory Th17 cells and anti-inflammatory Treg cells balance, thus contributing towards improvement in osteoporosis condition thereby reducing fracture risk (Lyu et al., 2023).
Rickets in children and osteomalacia in adults is a condition marked by the softening of bones due to insufficient bone mineralization from deficiencies in vitamin D, calcium, or phosphate. Vitamin D, derived from UV-B exposure or diet, is converted in the liver to calcidiol and then to active calcitriol in the kidneys. Calcitriol enhances calcium and phosphate absorption, essential to the mineralization of bone. Calcium or low vitamin D reduces serum calcium, triggering increased PTH secretion, which boosts calcitriol production and bone resorption to maintain calcium levels, but increases phosphate excretion. Prolonged phosphate loss leads to hypophosphatemia, impairing bone growth and mineralization (Sahay and Sahay, 2012). Dairy restricted diet and high phytates are significant contributors in this regard, therefore intake of dairy products enriched with vitamin D can be a good strategy to improve this condition. Fermented dairy with its probiotic content (having phytase enzyme) can enhance the bioavailability of calcium depressed by phytate (Parvaneh et al., 2014; Uday and Högler, 2020).
Fermented Dairy as Positive Modulator of Bone Health
Although genetics does have a significant influence on bone growth, dietary factors have their own significance since a healthy diet ensures the expression of these traits. Bone health depends on nutrients such as calcium, proteins, vitamin D, and other minerals, and deficiencies in these can lead to bone disorders, even if there is genetic predisposition for strong bone development.
Dairy products intake affects positively on bone health by focusing on fracture risk, BMC, and BMD. Dairy products provide 50%–60% of daily calcium and 20%–30% of protein, essential for bone growth and maintenance, aiding in achieving maximal bone mass during development and lowering adult bone turnover (Rizzoli, 2022). Intervention trials indicate that children avoiding dairy have a higher fracture risk, while adults consuming dairy, particularly fermented products like yogurt, show a lower risk of hip fractures due to the combined benefits of calcium, protein, and probiotics that support bone metabolism. Attaining high peak bone mass through dairy consumption reduces fracture risk later in life, with modest BMD increases linked to significant reductions in fracture risk, especially in postmenopausal women. Furthermore, short-term trials demonstrate that dairy products can lower markers of bone resorption, such as CTX and PTH levels, benefiting both young and older adults.
Calcium is the most significant mineral in achieving healthy BMD, preventing bone loss and fractures (Rizzoli, 2014). Calcium intake is important to develop and maintain a healthy bone mass and composition. Increased bone mass density and skeletal growth were observed in the 6 and 9 years old offsprings of expecting mothers who took calcium supplements (Cole et al., 2009; Ganpule et al., 2006). Due to their high calcium content and efficient absorption rate, dairy products are thought to be the best dietary source of calcium and low cost. In addition, dairy products have higher levels of protein, magnesium, potassium, calcium, zinc, and phosphorus per calorie as compared to other foods (Caroli et al., 2011; Heaney, 2000). For example, 170 g of yogurt, on average, provides 231 mg of calcium. In this way, 3–4 servings of the yogurt in a day will satisfy Recommended Daily Intake (RDI). For comparison, one serving of yogurt provides calcium amount which is provided by 5–6 servings of vegetables or 10–12 servings of grain food. Thus, 20%–28% of RDI of protein and 52%–65% that of calcium is satisfied via dairy products (Fulgoni et al., 2004; U.S. Department of Agriculture, 2010). Dairy products have been considered as essential components of a healthy diet both in the West and Asia (Ge, 2011; Lee and Cho, 2017).
Protein intake in diet provides the body with amino acids that are crucial for stimulating IGF-I an osteotrophic hormone for bone formation, and making bone matrix (Heaney, 2009). Positive correlation has been reported between BMD/BMC and spontaneous protein intake in pubertal boys. Additionally, dairy products are a significant source of protein. One liter of milk have about 35 grams of proteins including whey and casein proteins (Rizzoli, 2008). Long term low milk intake can lead to smaller stature, increased fracture risk by a factor of 2.7 folds (Goulding et al., 2004; Konstantynowicz et al., 2007). Intuitively, protein intake has been associated with the reduced hip fracture risk in an observational study (Wengreen et al., 2004). Table 1 enlists the amount of different bone-related nutrients in 100 g servings of various dairy products.
Data from U.S. Department of Agriculture (2010).
Probiotic-based dairy products contain live beneficial microorganisms, mainly lactic acid bacteria like Lactobacillus and Bifidobacterium (Table 2), which provide health benefits when consumed in sufficient amounts (Jang et al., 2024a; Saez-Lara et al., 2015).
| Dairy type | Probiotic strain | Reference |
|---|---|---|
| Acidophilus milk | Lactobacillus acidophilus | Hati and Prajapati (2022) |
| Fermented milk | L. acidophilus; Lactobacillus rhamnosus | Beltrán-Barrientos et al. (2016); Hou et al. (2019) |
| Lactobacillus fermentum; Lactobacillus plantarum | Mendez Utz et al. (2019) | |
| Lactobacillus paracasei; Lactobacillus casei | Oliveira et al. (2017) | |
| Lactobacillus delbruekii; Levilactobacillus brevis Streptococcus thermophilus; Bifidobacterium bifidum | Wa et al. (2019) | |
| Kefir | L. casei; L. acidophilus; L. paracasei; L. fermentum | Bengoa et al. (2019); Egea et al. (2022) |
| Kumys | Lactobacillus delbrueckii; Kluyveromyces marxianus | Arslan (2015) |
| Yogurt | L. acidophilus; Lactobacillus bulgaricus | Arain et al. (2023) |
| L. rhamnosus; L. plantarum | Ghasempour et al. (2020) | |
| Lactobacillus helveticus; L. casei | Lim et al. (2020) | |
| L. fermentum; S. thermophilus | Olson and Aryana (2022) | |
| Non-fermented Milk drink | L. plantarum | Jang et al. (2022) |
| Bifidobacterium lactis; Bifidobacterium animalis | Oliveira et al. (2017) |
Enhanced nutrient uptake: Several probiotics have been found to enhance bone growth, mineralization, and bone structure in animal models like rodents. Lactobacillus and Bifidobacterium species are the most common species of probiotics obtained from dairy products and they can improve bone health through different mechanisms which remain the subject of ongoing research, but some researchers suggest that they exert beneficial effect on bones through nutrient uptake, they enhance the absorption of vitamin D and other minerals by human and mouse epithelial cells (Wu et al., 2015). The possible mechanism involves further steps (a) probiotics and their secreted factors interact with the intestinal epithelial barrier and the cells located within the lamina propria. Within the lamina propria, probiotics and their secreted factors interact with antigen-presenting cells, including dendritic cells, to modulate the immune response. This interaction leads to a decrease in inflammatory cytokines, which in turn improves the absorption of minerals from the intestinal lumen. The factors secreted by bacteria subsequently enter the bloodstream and are transported to the bone, where they can interact with osteoclasts, osteoblasts, and immune cells. This may result in a reduction of pro-inflammatory and pro-osteoclastogenic cytokines, along with decreased oxidative stress, while simultaneously promoting mineral apposition and enhancing Wnt10b expression. This modulation reduces the formation of osteoclasts, which subsequently contributes to increased bone density (Collins et al., 2017). Probiotics are capable of digesting complex carbohydrates and producing oligosaccharides that can be further metabolized by other bacteria. This process promotes the proliferation of these bacteria and alters the composition of the microbiota.
Antimicrobial compounds and other peptides secretion: probiotic bacteria also produce antimicrobial agents that can target and eliminate specific bacterial strains in the gastrointestinal tract responsible for dysbiosis. For instance, lactobacilli strains produce lactic acid, bacteriocins (antimicrobial peptides) and hydrogen peroxide (Lebeer et al., 2008) and specifically, Lactobacillus helveticus synthesizes proline-rich peptides, namely isoleucyl-prolyl-proline (IPP) and valyl-prolyl-proline (VPP), which may enhance the bioavailability of minerals. Additionally, some peptides may not be directly absorbed but can aid in releasing minerals from insoluble compounds, thereby enhancing their absorption. Furthermore, the peptides IPP and VPP may function by inhibiting the conversion of Angiotensin I (Ang I) to Angiotensin II (Ang II; Parvaneh et al., 2014).
Immune modulation: It is well established that osteoclasts, which originate from monocytic precursors in the bone marrow, can interact with and be regulated by immune cells, such as B and T cells, as well as immune-stimulating factors like RANKL, TNF-α, IL-1, and IL-6 (Lorenzo et al., 2008). Probiotics seem to play a role in promoting bone health by influencing intestinal conditions by immunoregulation. In a study involving healthy male mice, treatment with Lactobacillus reuteri ATCC 6475, a candidate probiotic known for its anti-TNF-α activity, led to increased bone density. This enhancement was linked to lower levels of intestinal TNF-α, suggesting that probiotics can help prevent bone loss caused by inflammation (McCabe et al., 2013). However, it’s important to note that these positive effects on reducing inflammation and boosting bone density were only observed in male mice, indicating that the impact of probiotics may differ between genders. Another study showed that Lactobacillus plantarum A41 and Lactobacillusfermentum SRK414 exhibited significant antioxidant activity and a strong adhesion rate to intestinal cells. Furthermore, both L. plantarum A41 and L. fermentum SRK414 were found to reduce the mRNA expression levels of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-8. Furthermore, these strains were found to have the potential to mitigate diseases associated with bone loss by enhancing the mRNA expression of markers related to bone metabolism (Lee and Kim, 2020).
Production of SCFAs: Emerging research indicates that probiotics influence bone health through various mechanisms, including the production of SCFAs. Preclinical studies suggest that SCFAs, including butyrate and propionate, help prevent bone loss in osteoporosis models by inhibiting osteoclastogenesis and modulating the immune response (Chang et al., 2021; Feng et al., 2024). Administering SCFAs to mice, along with a high-fiber diet, significantly enhanced bone mass and prevented bone loss associated with postmenopausal conditions and inflammation. The protective effects of SCFAs on bone mass are linked to the inhibition of osteoclast differentiation and bone resorption both in vitro and in vivo, while bone formation remains unaffected (Lucas et al., 2018).
Hormone modulation: Estrogen and androgen, which are types of gonadal steroids, are essential in regulating bone metabolism by influencing bone mass and turnover (Xu et al., 2017). The decline in estrogen levels is a key factor contributing to the risk of postmenopausal osteoporosis. During menopause, the reduction in circulating estrogen can lead to various negative health outcomes, most notably a rapid loss of bone mass (Freedman, 2002). In the human body, estrogens are found in the bloodstream either in a free form or bound to proteins, where they exert a range of biological effects. Only the free form of estrogen is biologically active, whereas conjugated estrogens are inactivated and ultimately excreted in urine or feces. On the other hand, conjugated estrogen could be deconjugated by gut microbiome and probiotics (Kwa et al., 2016). It is well recognized that gut microbiota influences the absorption and metabolism of phytoestrogens, including isoflavones and lignans. Numerous phytoestrogens are hydrolyzed by intestinal microbes, such as Lactobacillus and Bifidobacterium species, resulting in the production of active compounds that improve their bioavailability (Xu et al., 1995). Recent studies have also investigated the potential effects of probiotic treatment in osteoporosis, particularly using the estrogen deficiency ovariectomized model. Previous studies indicated that healthy female mice did not show significant changes when supplemented with L. reuteri. However, L. reuteri was found to prevent bone loss induced by ovariectomy (OVX) in female mice, indicating its potential for preventing estrogen-deficiency-related osteoporosis in postmenopausal women (McCabe et al., 2013). Similar findings have been confirmed by other studies using comparable probiotic strains. Additionally, Lactobacillus paracasei has been demonstrated to prevent cortical bone loss induced by OVX and to reduce bone resorption (Chen et al., 2023; Collins et al., 2017; Ohlsson et al., 2014). Longitudinal bone growth is controlled by growth hormones (GH) and IGFs. These factors are crucial for cell survival, proliferation, and differentiation, and IGF-1 is considered a significant player in osteoblastogenesis. The bone quality is directly linked to serum IGF-1 level (Delagrange et al., 2021; Wong et al., 2016). Probiotics have been shown to impact the secretion of GH from the endocrine system, thereby contributing to the regulation of growth (Tu et al., 2023). Studies on germ-free mice revealed significantly lower levels of GH and IGF-1 compared to wild-type mice, but supplementation with L. plantarum restored IGF-1 levels to those of wild-type mice (Schwarzer et al., 2016). Probiotics secreted bacterial extracellular vesicles are an emerging new promising platform in bone health therapeutics along with their other biomedical applications. One such example is Akkermansia muciniphila derived EV which showed promising bone health improvement results as reported by Liu and colleagues (Liu et al., 2021). Fig. 2 summarizes the key ways through which probiotics improve bone health.
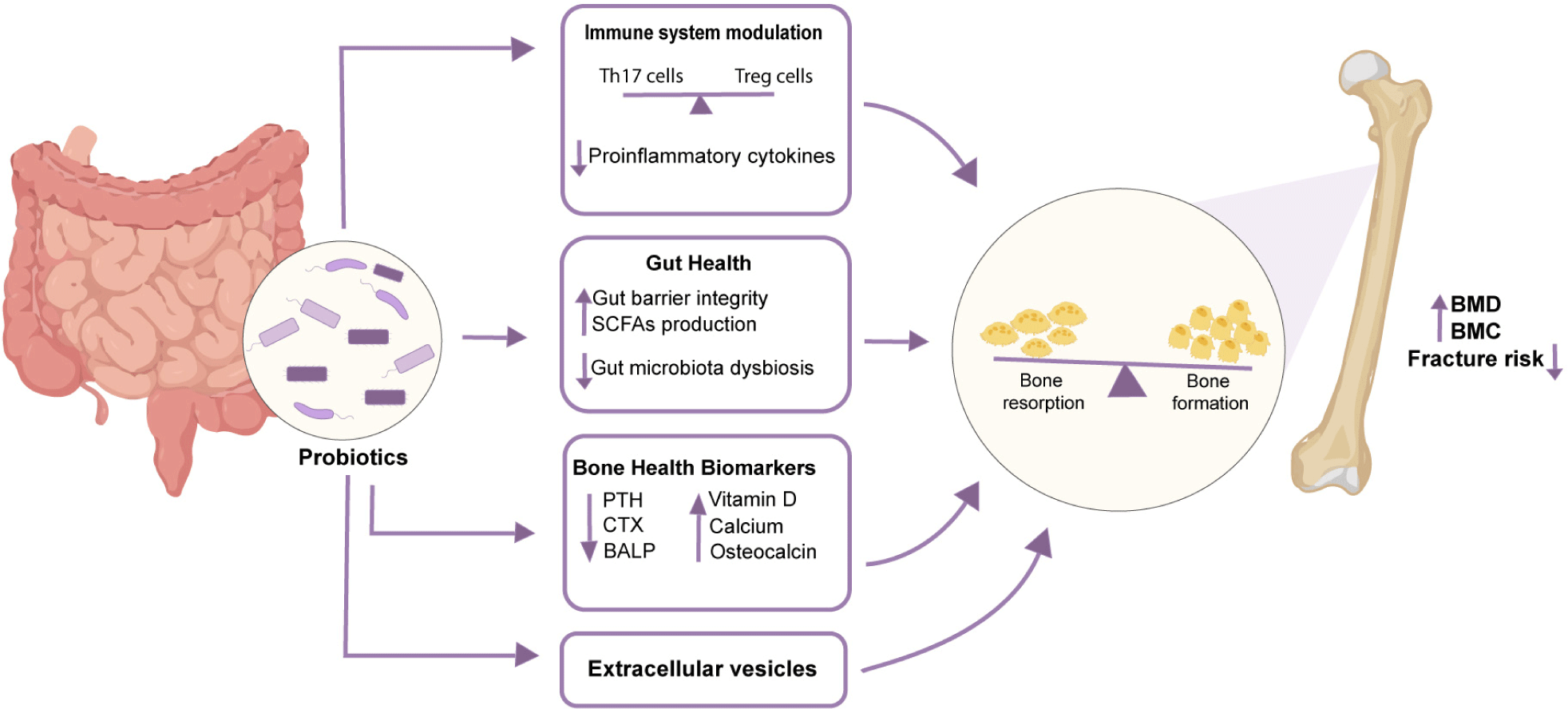
Probiotic strains, which have been extensively studied for their potential to enhance bone health, vary significantly in origin and functionality. Several common probiotics, along with their respective effects on bone health across different animal models, are summarized in Table 3. In addition, several studies have reported encouraging results of probiotics treatment on humans also as probiotics treatment reduced the bone resorption biomarkers and improved BMD in specific bone types (Table 4). One recent study, however, reported no significant bone health improvement after probiotics treatment (Sergeev et al., 2020). This disparity emphasizes the complexity of bone health regulation and the demand for more thorough research. Thus, Future research should concentrate on elucidating the mechanisms by which probiotics affect bone remodeling, considering differences in strain-specific effectiveness, and assessing the impact of host variables including dietary consumption, microbiome composition, and baseline bone health. Furthermore, to demonstrate the consistency and generalizability of the observed results, extended study periods and standardized procedures along with the use of better techniques are essential to translate the therapeutic potential of probiotics into reality.
| Probiotic strains | Animal model | Effects on bone health | References |
|---|---|---|---|
| Akkermansia muciniphila | Female mice (ovariectomy, OVX) | ↑ Osteocalcin (OCN) ↑ Bone formation rate per bone surface (BFR/BS) ↑ Mineral apposition rate (MAR) ↓ C-terminal telopeptides of type I collagen (CTX-I) ↓ Tartrate-resistant acid phosphatase (TRAP) activity |
Liu et al. (2021) |
| Bifidobacterium longum ATCC 15707 | Wistar rat | ↑ Tibial calcium, phosphorus, magnesium content | Rodrigues et al. (2012) |
| Lactobacillus reuteri ATCC 6475 | Male mice | ↑ Trabecular bone parameters ↑ Osteoblast serum markers and dynamic measures of bone formation |
McCabe et al. (2013) |
| Lactobacillus casei 393-fermented milk | Female Sprague-Dawlry rats (OVX) | ↑ Bone mineral density (BMD) ↑ Fracture strength ↑ Ca content |
Kim et al. (2009) |
| Lactobacillus helveticus-fermented milk | Spontaneously hypertensive male rats | ↑ BMD ↑ Bone mineral content |
Narva et al. (2004a) |
| Lactobacillus rhamnosus HN001 | Male Sprague-Dawlry rat | ↑ Ca and Mg retention | Kruger et al. (2009) |
| L. rhamnosus GG ATCC 53103 | C57BL6/J Mic | ↑ Trabecular bone microarchitecture, cortical bone volume and biomechanical properties | Liu et al. (2019) |
| Lactobacillus paracasei and Lactobacillus plantarum | Female mice (OVX) | ↑ BMD and the microstructure of femoral bone | Chiang and Pan (2011) |
| L. casei and Lactobacillus acidophilus | Wistar rat | ↓ Bone damage | Amdekar et al. (2012) |
| Lacticaseibacillus paracasei DSM13434, Lactiplantibacillus plantarum DSM 15312 | Female mice | ↑ Trabecular thickness in the proximal metaphyseal region of tibia | Ohlsson et al. (2021) |
| Probiotic strain | Type of study | Positive impact on bone health | Reference |
|---|---|---|---|
| Lactobacillus fermentum SRK414 | 6 mon randomized, double-blind, placebo-controlled trial | ↑ Femur, neck bone mineral density (BMD); maintenance of osteocalcin (OC) levels, indicating improved bone turnover | Han et al. (2022) |
| Bacillus subtilis C-3102 | 6 mon randomized, placebo-controlled, double-blind clinical trial | ↑ Hip BMD, ↓ urinary type I collagen cross-linked N-telopeptide (uNTx), ↓ tartrate-resistant acid phosphatase isoform 5b (TRACP-5b), modulation of gut microbiota | Takimoto et al. (2018) |
| Lactobacillus reuteri ATCCPTA 6475 | 12 mon randomized, placebo-controlled, double-blind clinical trial | ↓ Loss of tibia volumetric BMD; protective effects on bone microarchitecture | Nilsson et al. (2018) |
| Lactobacillus casei, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Streptococcus thermophilus | 6 mon randomized, double-blind, placebo-controlled clinical trial | ↓ Bone turnover markers (BALP, CTX) and pro-inflammatory cytokine; slowed bone turnover, beneficial for osteopenic postmenopausal women | Jafarnejad et al. (2017) |
| Proprietary lactic acid bacteria culture in red clover extract (RLE) | 12 mon double-blind, placebo-controlled randomized trial | ↓ BMD loss in lumbar spine, femoral neck, and trochanter; ↓ CTX; improved estrogen metabolism | Lambert et al. (2017) |
| L. reuteri NCIMB 30242 | 13 wk double-blind, placebo-controlled, randomized trial | ↑ Serum 25-hydroxyvitamin D levels by 25.5% | Jones et al. (2013) |
| Lactobacillus helveticus LBK-16H (fermented milk) | 1 mon randomized, double-blind, crossover clinical study | ↓ Serum parathyroid hormone levels ↑ Serum calcium concentrations |
Narva et al. (2004b) |
The probiotic adjuvant therapies in bone diseases show positive effect on patients. Recent study revealed that, supplementation with a multispecies probiotic for 12 weeks in osteopenic postmenopausal women may slow the rise in the serum bone resorption marker CTX by downregulating osteoclast-mediated bone resorption, without causing significant adverse effects (Vanitchanont et al., 2024). Probiotics and isoflavones are also believed to support bone health by influencing calcium absorption, gut microbiota, and various metabolic pathways linked to osteoblast activity and bone formation (Harahap and Suliburska, 2021). Overall, there is some evidence from human randomized controlled tests that probiotic supplementation can improve antioxidant defenses, disease activity and the inflammatory status of rheumatoid arthritis along with conventional medicines. Since probiotics have been shown to positively impact bone health, it suggests that combining them, such as using multispecies probiotics along with food and conventional medicines, could be a highly effective approach for addressing bone-related issues.
Conclusion
Consumption of fermented dairy products is significant to develop and maintain healthy bones in early and late phases of life, respectively. These products provide essential bone nutrients such as minerals, proteins as well as probiotics, which help reduce bone resorption and regulate the bone metabolism positively. Bone health-related nutrients, i.e. proteins and essential minerals are not only present in high concentration but also are available in more readily bio-available form in fermented dairy products. Dairy can provide up to 60% of daily calcium and 30% that of proteins. Probiotics contents of fermented dairy, in particular, help enhance calcium bioavailability and absorption, significantly bone resorption biomarkers and promote beneficial changes in the gut microbiome, all of which collectively contribute to healthier, stronger bones having enhanced BMD as well as BMC. Thus, incorporating fermented dairy products into the regular diet is an effective strategy to reduce bone-related disorders and fracture risk especially in aging populations.













