Introduction
Whey a by-product of cheese production in the dairy industry, often regarded as waste has an estimated global production of approximately 153 million tons annually (Kadyan et al., 2021). However, whey is now recognized as a rich source of bioactive components, including α-lactalbumin, β-lactoglobulin, glycomacropeptide, and immunoglobulins (Song et al., 2022). Recently, in the food industry, innovative products with higher functional value compared to that of traditional options are continually developed, including those fortified with whey (Purkiewicz and Pietrzak-Fiećko, 2021). Ahmadi et al. (2020) demonstrated that a whey beverage fortified with magnesium and vitamin C reduces inflammatory cytokine levels while improving skeletal muscle mass and strength indices. Moreover, homemade whey beverages enriched with up to 50% fruit and vegetable components exhibit significantly higher antioxidant activity and increased levels of phenolic compounds, flavonoids, and lipophilic pigments (Purkiewicz and Pietrzak-Fiećko, 2021). Nonetheless, investigation on the antibacterial and anti-biofilm effects in fortified whey beverages remains insufficient.
Triticum dicoccum, commonly referred to as “farro”, is among the most ancient wheat species in the world (Arzani and Ashraf, 2017; Padulosi et al., 1996). While being largely replaced by higher-yielding wheat varieties, such as Triticum aestivum (common wheat), by the early 20th century, interest in T. dicoccum wheat has recently grown. This resurgence is attributed to its superior nutritional profile, including lower starch digestibility, higher antioxidant content, and abundant dietary fiber, all of which are associated with significant health benefits (Dhanavath and Prasada Rao, 2017). T. dicoccum is particularly known for its high concentrations of proteins, dietary fiber, and antioxidant compounds (Lachman et al., 2012). Additionally, its lower starch digestibility makes it particularly suitable for patients with diabetes. Studies show that it can reduce glycemic response in type 2 diabetes models and significantly lower total cholesterol, triglycerides, and low-density lipoprotein-cholesterol levels compared to that of modern wheat diets (Yenagi et al., 2001). However, research on its antibacterial and anti-biofilm activities against foodborne pathogens remains largely limited.
The fermentation processes of grains have recently emerged as a promising technological approach (Gabriele and Pucci, 2022). The fermentation process alters the chemical composition of grains, enhances the levels of functional compounds, and further boosts the health benefits of the resulting products (Verni et al., 2019). The fermentation of Oryza sativa (indica rice) extract with lactic acid bacteria enhances its antibacterial and anti-biofilm effects against foodborne pathogens, including Salmonella Typhimurium, Yersinia enterocolitica, and Staphylococcus aureus (Kwon et al., 2022). Additionally, a study shows that sourdough fermentation enhances the nutraceutical and functional properties of spelt (Gabriele et al., 2023), supporting its potential therapeutic applications in managing hypertension, oxidative stress and inflammatory intestinal diseases.
Salmonella is a Gram-negative, rod-shaped bacterium recognized as a major causative agent of acute gastroenteritis (Seo and Kang, 2020). Globally, nontyphoidal Salmonella is estimated to be responsible for 93,757,000 cases of gastroenteritis annually, leading to approximately 155,000 deaths annually (Majowicz et al., 2010). Salmonella can also be found in various dairy products and dairy herds on farms (Adhikari et al., 2009; Sánchez-Vargas et al., 2011). In food processing environments, biofilms formed by Salmonella represent a contamination critical point in the food chain (Soni et al., 2013). Biofilm formation plays an essential role in the survival of bacteria within feed factory environments (Vestby et al., 2009). Therefore, the persistence of Salmonella significantly increases the risk of infection, particularly in environments that support bacterial survival. Addressing this issue necessitates a strategic focus on inhibiting biofilm formation, which serves as a protective mechanism for bacteria and an essential factor in its resistance to environmental stressors and sanitation measures.
Consequently, this study aimed to evaluate the effects of whey beverages supplemented with enzyme-rich fermented farro (referred to as “farro enzyme”) in inhibiting S. Typhimurium biofilm formation. Additionally, the study investigated the inhibitory effect of whey beverage supplemented with farro enzyme on the adhesion and invasion of S. Typhimurium in human intestinal epithelial cells.
Materials and Methods
The whey beverage was prepared following the method previously described (Song et al., 2022), with a few modifications. Whey powder was obtained from Samik Dairy and Food (Seoul, Korea) and suspended with distilled water in a 1:10 ratio. The whey suspension was sterilized by stirring in a water bath at 80°C for 3 min. The sterilized whey suspension was then mixed with 1% and 10% farro enzyme (v/v), which was provided from GrainOn (Seoul, Korea), and subjected to a secondary sterilization process at 60°C for 5 h. The mixture was then centrifuged at 15,000×g for 20 min, and the resulting supernatants were adjusted to a pH 7.2–7.4 using NaOH and HCl. The whey beverage was then filtered through a 0.2 μm syringe filter.
S. Typhimurium KCTC 1925 was obtained from the Korean Collection for Type Cultures (Jeongup, Korea) and incubated in Luria–Bertani (LB) medium (LPS solution, Daejeon, Korea) at 37°C. S. Typhimurium was incubated for 15 h at 37°C and adjusted to 2×107 CFU/mL using LB broth. Subsequently, 100 μL of bacterial suspension was added to each well of a 96-well plate, along with 100 μL of whey beverage supplemented with 1% farro enzyme (WF1) or 10% farro enzyme (WF10). The plate was incubated at 37°C for 24 h. Additionally, the bacterial suspension was also incubated with whey beverage without farro enzyme (WF0) under the same condition as a control. In a separate experiment, a 96-well plate was pretreated with 100 μL of WF0, WF1, or WF10 for 24 h. Following this, 100 μL of S. Typhimurium (2×107 CFU/mL) was added and incubated at 37°C for an additional 24 h. Conversely, 100 μL of S. Typhimurium (2×107 CFU/mL) was preincubated at 37°C for 24 h in a 96-well plate and then treated with 100 μL of WF0, WF1, or WF10 for 24 h. Following this, the culture supernatants were discarded, and the culture plates were gently rinsed with phosphate-buffered saline (PBS). S. Typhimurium biofilm was stained with 0.1% crystal violet solution for 30 min at 25°C, then washed with PBS to remove excess dye. The bound stain was resolubilized in a solution of 0.1% acetic acid and 95% ethanol, and absorbance was measured at 595 nm using a microplate reader (AMR-100, Allsheng, Hangzhou, China).
After confocal dishes were pretreated with 100 μL of WF0, WF1, or WF10 at 37°C for 24 h, 100 μL of S. Typhimurium culture (2×107 CFU/mL) was added and incubated at 37°C for an additional 24 h. After discarding the culture supernatants, the confocal dishes were gently washed with PBS. The S. Typhimurium biofilm was stained using a LIVE/DEAD BacLight bacterial viability kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s instructions and observed using a confocal laser scanning microscope (Eclipse Ti-E, Nikon, Tokyo, Japan) at ×600 magnification.
To assess the viability of planktonic S. Typhimurium, a 96-well plate was pretreated with 100 μL of WF0, WF1, or WF10 at 37°C for 24 h. S. Typhimurium culture was then added and incubated at 37°C for an additional 24 h. Following incubation, the supernatants were harvested to obtain planktonic S. Typhimurium, which was then serially diluted in PBS. The viability of planktonic S. Typhimurium was determined by plating on LB agar.
The adhesion and invasion assay were conducted following the method as previously described (Lee et al., 2022), with some modifications. The human intestinal epithelial cell line HT-29 was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Welgene, Gyeongsan, Korea) containing 10% fetal bovine serum (FBS; Gibco, Burlington, ON, Canada), 100 U/mL penicillin, and 100°Cμg/mL streptomycin (HyClone, Logan, UT, USA) at 37°C in a 5% CO2 atmosphere in a humidified incubator. To investigate the inhibitory effects of WF0, WF1, or WF10 on the adhesion of S. Typhimurium to HT-29 cells, HT-29 cells were plated in a 12-well plate at 5×105 cells/mL and grown to full confluence. S. Typhimurium cultures were washed with PBS and resuspended in antibiotic-free DMEM containing 10% FBS. The monolayer of HT-29 cells was carefully washed with PBS and subsequently cotreated with the bacterial culture in antibiotic-free DMEM and WF0, WF1, or WF10. Following a 1 h incubation with gentle shaking, the cells were carefully washed with PBS to remove non-adherent bacteria and treated with 0.2% Triton X-100 for 20 min to lyse the adherent bacteria. The adherent bacteria counts were determined by plate count method using LB agar. For the invasion assay, fully confluent HT-29 cells were treated with WF0, WF1, or WF10 and S. Typhimurium, as previously described above. After washing with PBS, the cells were treated with gentamycin (100 μg/mL) and suspended in antibiotic-free DMEM for 1 h to eliminate extracellular bacteria. The cells were then lysed with 0.2% Triton X-100, and the invasion of S. Typhimurium was quantified by plating on LB agar.
The inhibitory effect of WF0, WF1, or WF10 on S. Typhimurium biofilm formation was evaluated by analyzing the expression of biofilm-associated genes using quantitative reverse transcription polymerase chain reaction (qRT-PCR). Following the incubation of WF0, WF1, or WF10 in a 6-well plate for 24 h, S. Typhimurium culture was added in each well and incubated at 37°C for an additional 24 h to allow biofilm formation. The biofilm was detached by pipetting, transferred to a 1.7 mL microtube, and centrifuged at 15,000×g for 5 min. The resulting bacterial pellets were resuspended in 1.5 mg/mL lysozyme (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37°C for 30 min. Total RNA was extracted from S. Typhimurium using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and reverse transcription was conducted using random hexamers and M-MLV reverse transcriptase (Promega, Madison, WI, USA) to synthesize complementary DNA (cDNA). The resulting cDNA was then amplified using the SYBR Green Real-Time PCR Master Mix (Toyobo, Osaka, Japan) on a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The PCR conditions included an initial denaturation at 95°C for 10 s, followed by 40 cycles of 95°C for 5 s and annealing/extension at 60°C for 31 s. Relative gene expression was quantified using the 2-ΔΔCt method, with normalization to 16S rRNA. The sequences of primers used in this study are presented in Table 1.
To determine autoinducer-2 (AI-2) signaling molecule production of S. Typhimurium, a bioluminescence assay was conducted with some modifications to the methodology as previously described (Taga and Xavier, 2011; Wattanavanitchakorn et al., 2014). S. Typhimurium cultures were treated with or without WF0, WF1, or WF10 and incubated at 37°C for 24 h. Following incubation, cell-free supernatants were collected by centrifugation at 12,000×g for 10 min and subsequently filtered through a 0.2 μm membrane. The AI-2 reporter strain, Vibrio campbellii BB-170, was cultured overnight in an autoinducer bioassay (AB) medium at 30°C. This culture was subsequently diluted 1:5,000 in fresh AB medium. The diluted V. campbellii BB-170 culture was combined with the cell-free supernatants at a 1:9 ratio and incubated at 30°C for 7 h. Bioluminescence was then measured using a Victor X4 Multimode Plate Reader (PerkinElmer, Waltham, MA, USA). The relative AI-2 production was calculated as a percentage compared to that of the untreated control.
All data in this study are presented as the mean±SD from triplicate experiments. Statistical analysis was conducted to compare the results of each assay to that of the respective control group. Statistical significance (p<0.05) was determined using an unpaired two-tailed t-test (GraphPad Prism 5, GraphPad Software, La Jolla, CA, USA) or one-way analysis of variance (ANOVA; IBM SPSS Statistics 23, IBM, Armonk, NY, USA).
Results
The inhibitory effect of whey beverages on S. Typhimurium biofilm formation was evaluated using crystal violet staining. The whey beverage was observed to effectively inhibit biofilm formation in a farro enzyme concentration-dependent manner when applied as a pretreatment and cotreatment. Fig. 1A shows that biofilm formation was decreased by approximately 19% and 31% when pretreated with WF1 and WF10, respectively, compared to that of the untreated control. Additionally, when cotreated with WF1 and WF10, biofilm formation was significantly reduced by approximately 22% and 60%, respectively, compared to that of pretreatment (p<0.05; Fig. 1B). However, WF0 was unable to inhibit S. Typhimurium biofilm formation in pretreatment and cotreatment conditions (p>0.05). Posttreatment with WF0, WF1, and WF10 did not consistently reduce biofilm formation (Fig. 1C). Confocal laser scanning microscopy further confirmed the concentration-dependent inhibitory effect of farro enzyme supplementation during cotreatment with WF1 and WF10 on S. Typhimurium biofilm formation. Untreated S. Typhimurium samples demonstrated a dense, thick, and fully matured biofilm, whereas samples treated with WF10 exhibited significantly less dense biofilms, characterized by dispersed individual colonies. These findings indicate the significant inhibitory effect of whey beverage supplemented with farro enzyme in disrupting S. Typhimurium biofilm formation (Fig. 2).

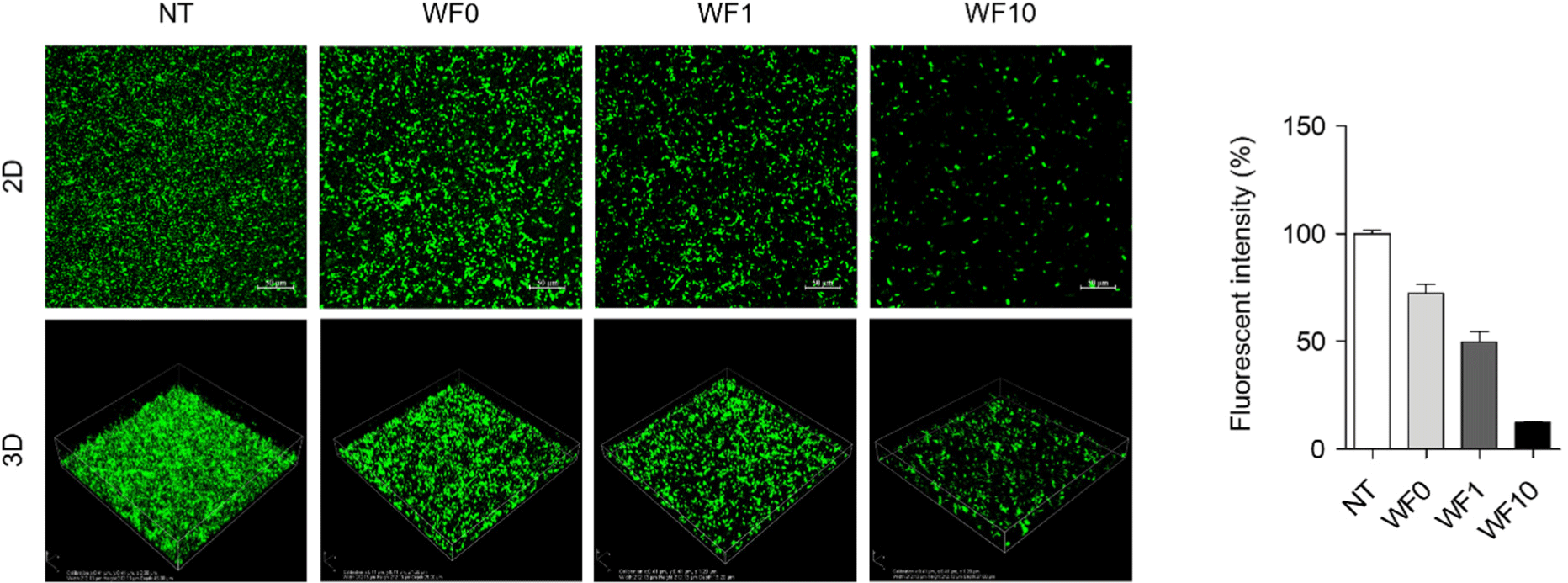
To evaluate whether whey beverage supplemented with farro enzyme inhibits S. Typhimurium biofilm formation by reducing bacterial viability in the planktonic state, the viability of planktonic S. Typhimurium cultured with WF0, WF1, or WF10 was quantified. Fig. 3 shows a concentration-dependent decrease in the viability of planktonic S. Typhimurium with increasing concentrations of farro enzyme. Incubation with WF10 for 24 h significantly reduced the viability of planktonic S. Typhimurium by approximately 88%.
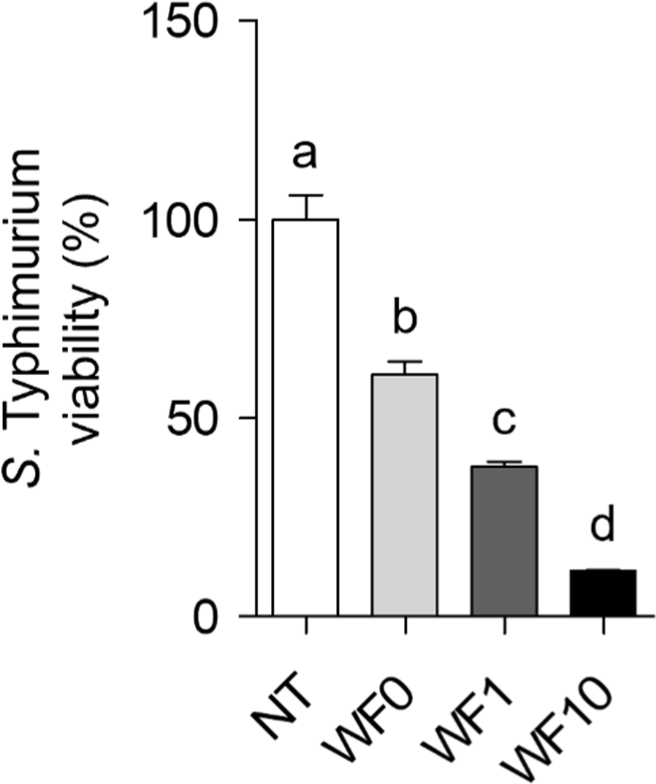
Fig. 4A shows that the adhesion of S. Typhimurium to HT-29 cells was significantly inhibited by WF1 and WF10 compared to that of WF0 (74% inhibition), with reductions of 44% and 32%, respectively. Similarly, Fig. 4B shows that the invasion of S. Typhimurium into HT-29 cells was not significantly reduced by WF0. However, WF1 and WF10 (47% and 17%, respectively) significantly suppressed the invasion of S. Typhimurium into HT-29 cells in a concentration-dependent manner with respect to farro enzyme supplementation.
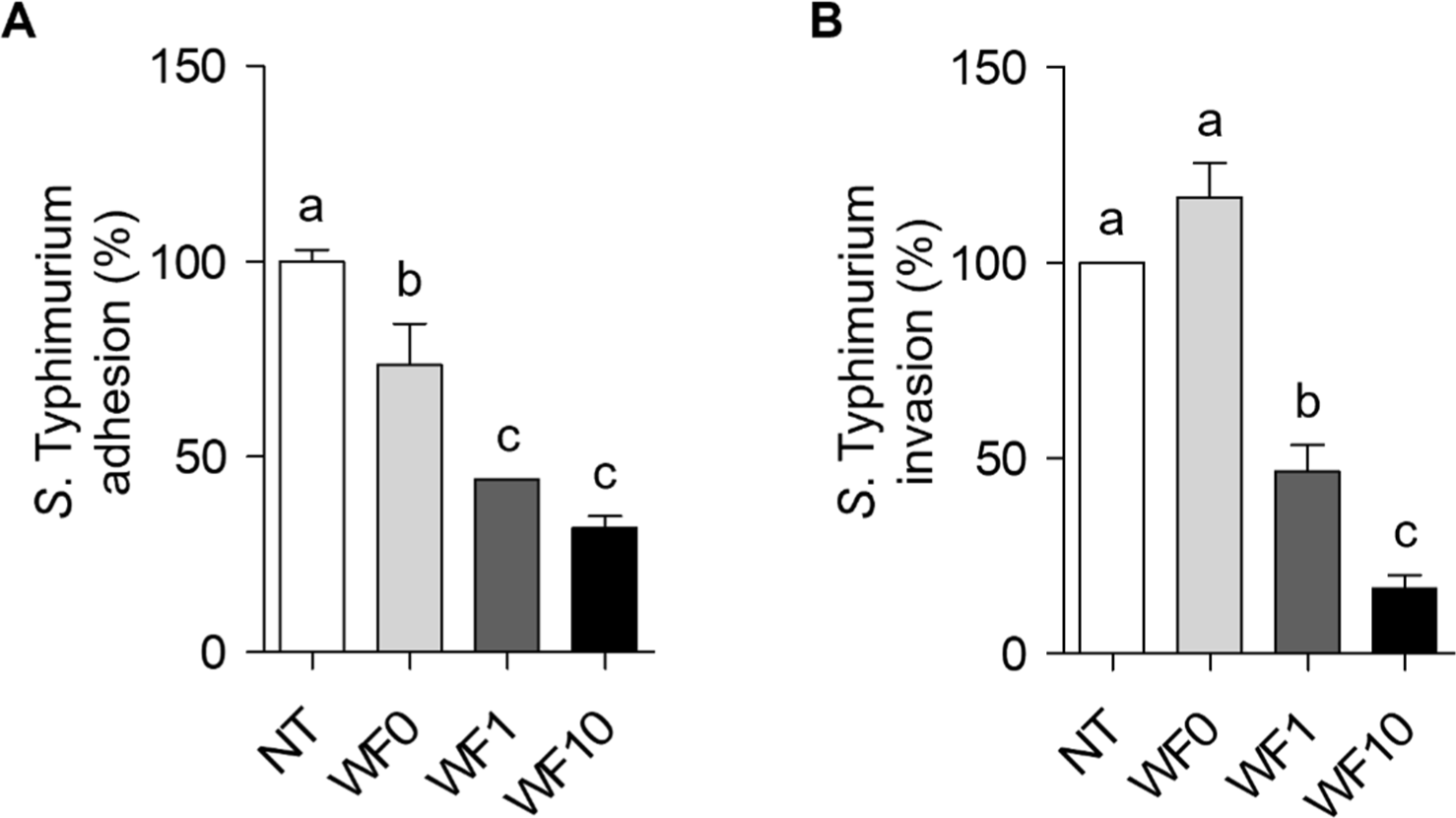
To investigate the gene regulation involved in S. Typhimurium biofilm formation in the presence or absence of WF0, WF1, and WF10, the transcriptional profiles of filC, lsrD, adrA, sdiA, luxS, and csgD were analyzed. The expression levels of genes associated with adhesion and biofilm formation, such as filC and csgD, were significantly reduced by >80% in S. Typhimurium treated with WF0, WF1, and WF10, compared to that of the untreated control (Fig. 5). Additionally, gene expression levels associated with QS and regulation, such as lsrD and luxS, as well as those associated with virulence and motility, including adrA and sdiA, were significantly decreased. The reduction in gene expression by at least 80% is considered to correlate with the reduction in the viability of planktonic S. Typhimurium following treatment with WF0, WF1, and WF10. A significant reduction of filC, lsrD, adrA, sdiA, and luxS in a supplemented farro enzyme concentration-dependent manner was observed (p<0.05). Although not statistically significant, a concentration-dependent decrease in csgD gene expression was observed with farro enzyme supplementation (p>0.05).
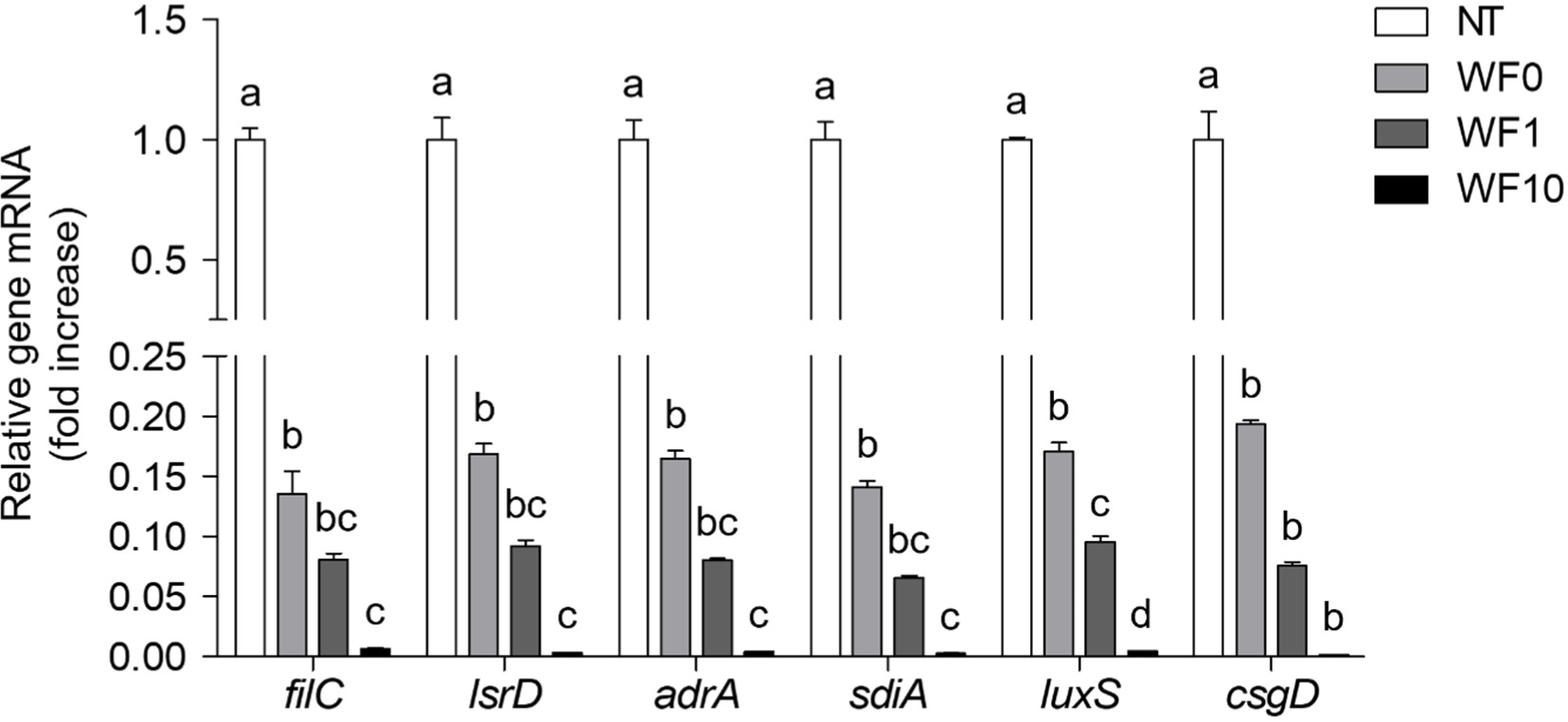
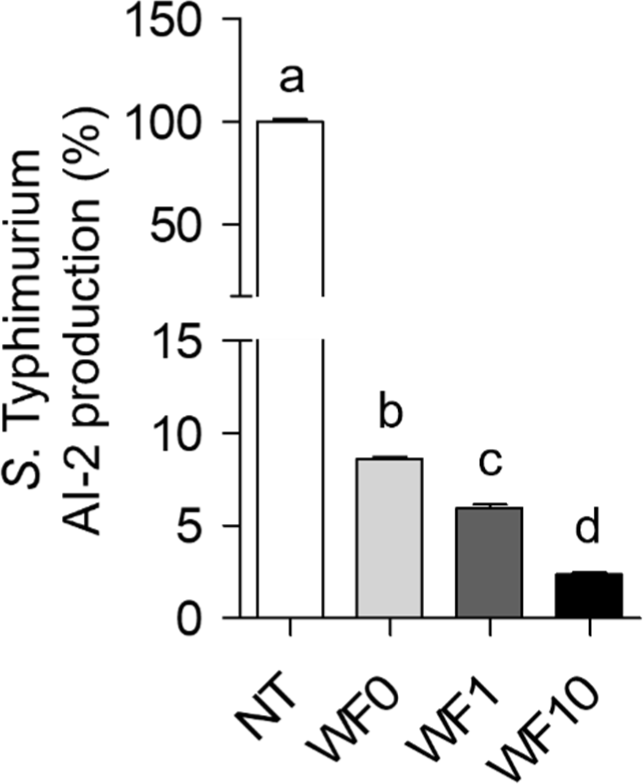
To assess whether QS is involved in the reduction of S. Typhimurium biofilm formation, AI-2 production by V. campbellii BB-170 was measured after treatment WF0, WF1, and WF10. Fig. 6 shows that WF0, WF1, and WF10 treatment demonstrated a concentration-dependent inhibitory effect of AI-2 in a supplemented farro enzyme, reducing it by >90%. The reduction in AI-2 production by >90% is probably associated with the lowered viability of planktonic S. Typhimurium following WF0, WF1, and WF10 treatment. This finding suggests that whey beverage supplemented with farro enzyme inhibits QS by decreasing the AI-2 signaling molecule.
Discussion
S. Typhimurium is a major zoonotic pathogen responsible for foodborne outbreaks, with its AI-2/luxS QS system influencing virulence, biofilm formation, and food spoilage, ultimately leading to food poisoning and disease transmission (Nahar et al., 2021; Zhao et al., 2017). Previous studies show the antimicrobial activity of whey and its peptide against various foodborne pathogenic bacteria (Abdel-Hamid et al., 2016; Osman et al., 2016). Whey, a by-product of the dairy industry, contains several bioactive components, including branched-chain amino acids, essential amino acids, and functional peptides, and offers health benefits against various metabolic diseases (Boscaini et al., 2023; Patel, 2015). However, to date, the potential biofilm inhibitory effect of unmodified whey beverages remains uninvestigated. Our results demonstrated the potential supplementation of whey with farro enzyme in effectively inhibiting biofilm formation by reducing the adhesion and QS of S. Typhimurium along with decreasing its viability. This finding suggests that the functionality of whey can be enhanced by adding farro enzyme, offering the potential to expand the application of whey-based products and generate added value.
To our knowledge, this study is the first to report the anti-biofilm properties of whey beverages supplemented with T. dicoccum (farro enzyme). T. dicoccum, an ancient wheat species, has recently garnered attention for its numerous health benefits and suitability for organic farming practices (Dhanavath and Prasada Rao, 2017). Fermentation processes have been widely employed to enhance the nutritional and nutraceutical properties of plant-based foods (Garrido-Galand et al., 2021). A study shows that fermentation improves the functional properties of farro, including its anti-inflammatory and antioxidant activities (Gabriele et al., 2023). Another study shows that whole flours sourced from different species of Triticum genus exhibit antimicrobial activity against enteric pathogenic bacteria, including Escherichia coli and S. Typhimurium (Grande et al., 2023). Another related study shows the antimicrobial potential of a novel peptide WBp-1 from wheat bran (Zou et al., 2025). Furthermore, whey stimulates the growth of probiotic bacteria, demonstrating its potent prebiotic effect (Sánchez-Moya et al., 2017). Whey and butter milk-based formula also modulate gut microbiota by preventing or restoring antibiotic-induced alterations (Bellés et al., 2023). Thus, it is plausible that whey beverages supplemented with farro enzymes could also influence the intestinal microbiome. However, the antibacterial and antibiofilm activity of T. dicoccum remains extensively uninvestigated. Therefore, our study is the first to demonstrate that whey beverages supplemented with farro enzyme effectively inhibit S. Typhimurium biofilm formation, which also explains the underlying mechanisms involved.
The biofilm has the potential to serve as a virulence factor, playing an essential role in the pathogenic mechanisms of S. Typhimurium (Eran et al., 2020; Seo and Kang, 2020). The intestinal mucosa is a primary target for Salmonella, where, following ingestion, it traverses the gastrointestinal tract to adhere to and invade host tissues (Gagnon et al., 2013; Wagner and Hensel, 2011). These processes are essential steps in pathogenesis; therefore, inhibiting the adhesion and invasion of intestinal epithelial cells is crucial for preventing infection. The initial adhesion and early biofilm formation of Salmonella are primarily driven by flagella, with fliC encoding the major constituent of the flagellin structure, essential for forming the helical filament (Vilas Boas et al., 2024; Wang et al., 2020). Subsequently, csgD coregulates the synthesis of curli fimbriae, whereas the adrA gene controls the production of extracellular matrix components, such as cellulose, which contribute to biofilm formation and maintaining its structural integrity (González et al., 2024; Li et al., 2024). Our findings indicate that treatment with WF1 and WF10 inhibits adhesion and invasion of intestinal epithelial cells by reducing the expression of the flaA, csgD, and adrA genes. These findings correspond with that of Salehi et al. (2017), who demonstrated that flagellar ΔfliCΔfljB mutants exhibit reduced adhesion and invasion to intestinal epithelial cells.
Furthermore, the findings of this study indicate that treatment with WF1 and WF10 significantly suppressed the expression of additional QS-related genes in S. Typhimurium, including lsrD, sdiA, and luxS. QS is a bacterial cell-to-cell communication mechanism in which the production, release, and detection of signaling molecules, known as Ais, occur in response to fluctuations in population density. This process plays an essential role in the behavior of bacteria that cause foodborne diseases (Choi et al., 2012; Escobar-Muciño et al., 2022). QS is commonly targeted as a strategic mechanism for inhibiting biofilm formation (Koutsoudis et al., 2006). The inhibition of sdiA and luxS may be attributed to the suppression of signal molecule biosynthesis or interference with the reception of acyl-homoserine lactone. Alternatively, WF1 and WF10 might promote enzyme inactivation or degrade molecules involved in the QS system (Eran et al., 2020; Mi et al., 2024). 4,5-Dihydroxy-2,3-pentanedione, commonly referred to as “AI-2,” is a key compound in the QS pathway that exists in multiple forms. In S. Typhimurium, AI-2 plays a significant role in controlling cellular processes critical to pathogenesis, including the production of virulence factors and biofilm formation (Almasoud et al., 2016; Vijayababu et al., 2018). Our findings indicate that AI-2 production of S. Typhimurium treated with whey beverage supplemented with farro enzyme is reduced in a concentration-dependent manner. Our study revealed that AI-2 production in S. Typhimurium treated with WF1 and WF10 was reduced in a concentration-dependent manner with the supplementation of the farro enzyme. Consequently, the reduction in AI-2 production triggered by WF leads to a disruption in biofilm stability and a decrease in the virulence of S. Typhimurium by impairing its adhesion and motility.
Conclusion
This study highlights the potential of whey beverages supplemented with farro enzyme as a functional component for enhancing the antibacterial and anti-biofilm properties of whey beverage. The addition of whey beverage supplemented with farro enzyme significantly inhibited S. Typhimurium biofilm formation and reduced bacterial adhesion as well as invasion of intestinal epithelial cells. The concentration-dependent suppression of biofilm formation was associated with the downregulation of motility, adhesion, and QS-related genes, as well as inhibiting AI-2 production. These findings suggest that whey beverage supplemented with farro enzyme exerts anti-biofilm activity via multiple mechanisms, including antimicrobial action, interference with initial bacterial adhesion, and disrupting QS signaling. This research offers a promising foundation for developing fortified dairy beverages aimed at improving food safety and human health. However, challenges remain for practical application, maintaining sensory quality as drink, and confirming efficacy against a broader range of pathogens. Further research is needed to optimize formulation and evaluate the long-term health benefits of these functional beverages.













