Introduction
Food is not only an essential source of nourishment but also a source of pleasure, especially when it comes to meat. Meat contains several essential nutrients, including proteins, lipids, vitamins, and minerals (Biesalski, 2005). Several factors can influence meat quality during processing and storage and controlling these factors is important in the meat industry in order to ensure optimal quality and consumer satisfaction. Of the many quality properties of meat, those related to safety, particularly to pathogenic microorganism contamination, are of utmost concern. The high moisture content and abundance of nutrients found in meat results in favorable growth conditions for microorganisms (Alahakoon et al., 2015; Biesalski, 2005; Jayasena et al., 2015). While heat pasteurization is an effective way to kill these microorganisms in fresh meat, the high temperature results in undesirable changes in the meat's quality, including changes in its appearance, texture, and nutritional content (Awuah et al., 2007; Deng et al., 2007). Therefore, various technologies have been developed for pasteurization for raw meats. Irradiation is one common non-thermal pasteurization method. It uses a linear accelerator for electron beam irradiation and cobalt 60 for gamma irradiation (Ahn et al., 2016) and has outstanding efficacy (Kim et al., 2015). However, the construction and operation of irradiation facilities is costly, and it has been reported that irradiation can generate an off-flavor in the meat (Brewer, 2009). High pressure processing (HPP) technology is another effective non-thermal pasteurization method (Zhang and Mittal, 2008), but, HPP causes discoloration in fresh red meat and can alter its physiochemical, morphological, structural, and textural characteristics (Cheftel, 1995; Kim et al., 2007).
Plasma, especially cold plasma, is a relatively new method of non-thermal pasteurization under investigation for use in the food industry. Plasma is a form of ionized gas that is generated by applying an electric current to a neutral gas (Conrads and Schmidt, 2000). Plasma contains reactive oxygen species (ROS) and reactive nitrogen species (RNS) that cause oxidative damage to the outer membranes and DNA of microorganisms resulting in cell death (Afshari and Hosseini, 2014). Based on the properties of ROS and RNS in plasma, the microbicidal effects of plasma treatment in meat and meat products have been widely studied and reviewed (Mir et al., 2016; Misra and Jo, 2017). Recently, plasma has been shown to have the potential to be a source of nitrite, which is an important additive in the production of cured meat products (Jung et al., 2015b). In this review, we explore the potential applications of plasma for use in the meat and meat product processing industry as a non-thermal pasteurization method. Furthermore, the roles of plasma treatment as a curing for meat products are reviewed.
Atmospheric Pressure Cold Plasma
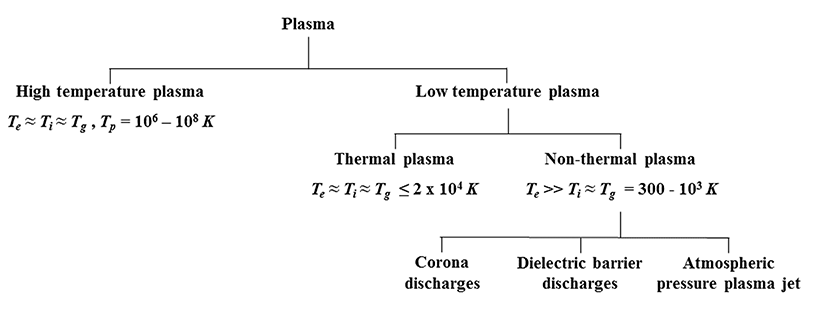
Plasma, which has been described as the fourth state of matter, is partially or fully ionized gas composed of positive and negative ions, electrons, free radicals, and neutral particles (Nehra et al., 2008). It is generated by applying an electric current across neutral gases, which results in the dissociation of the gaseous molecules (Conrads and Schmidt, 2000; Nehra et al., 2008). Plasma can be divided into two types based on temperature: high temperature plasma and low temperature plasma. High temperature plasma exists in a thermal equilibrium state in the range of 106 to 108K (Fig. 1; Nehra et al., 2008). Low temperature plasma can be further divided into thermal or nonthermal plasma. Thermal plasma exists in a local thermal equilibrium state with temperatures ranging from 4000 to 20,000 K (Bogaerts et al., 2002; Schluter et al., 2013). Non-thermal plasma, also known as cold plasma, exists in a non-equilibrium state with a temperature range of 300 to 1000 K (Nehra et al., 2008). High temperature and thermal plasmas are not suitable for use on heat sensitive foods because the heat transfer from the plasma to the food causes deterioration in the food's quality. Therefore, non-thermal plasma methods of pasteurization are of considerable interest to the meat industry.
Plasma can be generated over a wide range of pressures depending on the plasma source. In early studies, plasma was generated at low pressures (Napartovich, 2001). However, a vacuum system was required for plasma generation, and consequently, the applicability of low pressure discharge plasma is limited. For this reason, various other plasma sources, including dielectric barrier discharge (DBD), corona discharge, and atmospheric plasma jet, have been developed for plasma generation at atmospheric pressure (Nehra et al., 2008). DBD plasma generators are comprised of two electrodes; a high voltage electrode and a ground electrode. The gas in the gap between these electrodes undergoes electrical breakdown when a high-voltage electric current is applied (Kogelschatz, 2003). Furthermore, DBD plasma can easily be produced at scale in laboratory or industry (Kogelschatz, 2003).
Atmospheric pressure cold plasma (APCP) is the plasma generated at atmospheric pressure and its temperature is around 30-60°C (Misra et al., 2011). It can be produced with various plasma sources such as DBD, corona discharges, and atmospheric plasma jet, with various discharge gas such as nitrogen, oxygen, helium, and argon as well as ambient air (Nehra et al., 2008). Recently, APCP generated using a DBD system is of particular interest to the meat industry.
Application of Atmospheric Pressure Cold Plasma in the Meat Industry
Meat provides a favorable environment for the growth of pathogenic and spoilage microorganisms because of its high moisture content and abundance of nutrients (Alahakoon et al., 2015; Biesalski, 2005). Therefore, the elimination of pathogenic microorganisms from meat and meat products is important for consumer safety. Furthermore, increasing shelf life via the elimination of spoilage microorganisms in meat and meat products is important for food security and the economic viability of the meat industry. Non-thermal pasteurization is regarded as an ideal pasteurization method for meat and meat products because chemical pasteurization methods with chlorine, organic acids, peroxyacetic acid, and phosphate can leave behind harmful substances or deteriorate meat quality and thermal pasteurization results in a deterioration of meat quality (Chen et al., 2011).
Many previous studies have examined the applicability of APCP treatment as a technique for the non-thermal pasteurization of foods. ROS and RNS are produced via the dissociation of gaseous molecules during the plasma generation (Conrads and Schmidt, 2000; Han et al., 2016). ROS, including singlet oxygen, hydroxyl radical, superoxide anion, hydrogen peroxide, and ozone, in plasma have microbicidal properties, with ozone of particular importance because of its relatively long life time (Han et al., 2016; Laroussi and Leipold, 2004; Ziuzina et al., 2014). The ROS in APCP act via different microbicidal mechanisms on Gram-positive and Gram-negative bacteria. Gram-positive bacteria have a thick cell wall composed of peptidoglycans. Exposure of Listeria monocytogenes and Staphylococcus aureus to APCP causes cell shrinkage but little damage to the cell wall (Cullen et al., 2014; Han et al., 2016). Furthermore, intracellular ROS levels have been shown to increase in L. monocytogenes and S. aureus with prolonged exposure to APCP (Han et al., 2016; Ziuzina et al., 2015). It has also been reported that the ROS generated by DBD plasma can penetrate the cell membrane and lead to cell apoptosis via intracellular DNA damage (Sensenig et al., 2011). Therefore, the microbicidal effects of APCP on Gram-positive bacteria appear to be mainly the result of oxidative damage to intracellular components, particularly DNA (Han et al., 2016). The cell walls of Gram-negative bacteria consist of an outer membrane of lipopolysaccharide and a thin layer of peptidoglycan. Following APCP treatment, visible morphological changes, including cell breakage, have been observed along with an increase in cell leakage in Escherichia coli (Han et al., 2016). Previous studies have found that ROS attack and destroy the cell walls of Gram-negative bacteria by cleaving the C-O, C-N, and C-C bonds of the peptidoglycans and oxidizing the lipids in the lipopolysaccharides (Dobrynin et al., 2009; Han et al., 2016; Yusupov et al., 2013). While an increase in intracellular ROS level was found in E. coli following APCP treatment, this increase was less than in L. monocytogenes and S. aureus (Han et al., 2016; Ziuzina et al., 2015). Furthermore, the extent of DNA damage was less in E. coli than S. aureus (Han et al., 2016). Therefore, Han et al. (2016) proposed that the microbicidal effects of APCP treatment in Gram-negative bacteria is mainly caused by the destruction of the cell wall via oxidative damage.
The microbicidal effects of APCP generated using DBD on various microorganisms in meat and meat products have also been investigated (Table 1). Previous studies have shown than the levels of L. monocytogenes in inoculated meat and meat products were reduced as much as 0.59 to 6.52 Log CFU/g after plasma treatment (Jayasena et al., 2015; Kim et al., 2011; Kim et al., 2013; Lee et al., 2011; Yong et al., 2017a). Noriega et al. (2011) reported a 3.30 Log CFU/g reduction in L. innocua levels in inoculated chicken breast following plasma treatment. A 0.35 to 3.00 Log CFU/g reduction in E. coli and a 1.70 to 3.03 Log CFU/g reduction in S. Typhimurium levels in inoculated meat and meat products were also found after plasma treatment (Jayasena et al., 2015; Kim et al., 2011; Kim et al., 2013; Yong et al., 2017a). Furthermore, a 2.54 Log CFU/g reduction in S. enterica and a 2.45 Log CFU/g reduction in C. jejuni levels in chicken breast were found following plasma treatment (Dirks et al., 2012).
| Microbes | Reduction rate (Log CFU/g) / Substrate | Discharge gas | Power / Frequency | Exposure time | Type / Distance | Reference |
|---|---|---|---|---|---|---|
| Gram-positive | ||||||
| L. monocytogenes | 6.52 / ham | Nitrogen / oxygen | 2 kV / 50 kHz | 2 min | Closed / - | Lee et al., 2011 |
| L. monocytogenes | 4.73 / chicken breast | Nitrogen / oxygen | 2 kV / 50 kHz | 2 min | Closed / - | Lee et al., 2011 |
| L. monocytogenes | 2.60 / bacon | Helium / oxygen | 125 W | 90 s | Open / 3 mm | Kim et al., 2011 |
| L. monocytogenes | 0.59 / pork loin | Helium / oxygen | 3 kV / 30 kHz | 10 min | Open / 3 mm | Kim et al., 2013 |
| L. monocytogenes | 2.04 / pork butt | Nitrogen / oxygen | 100 W / 15 kHz | 10 min | Closed / - | Jayasena et al., 2015 |
| L. monocytogenes | 1.90 / beef loin | Nitrogen / oxygen | 100 W / 15 kHz | 10 min | Closed / - | Jayasena et al., 2015 |
| L. monocytogenes | 2.36 / beef jerky | Ambient air | 15 kHz | 10 min | Closed / - | Yong et al., 2017a |
| L. innocua | 3.30 / chicken breast | Oxygen / helium | 16 kV / 30 kHz | 8 min | Open / 1 cm | Noriega et al., 2011 |
| Gram-negative | ||||||
| E. coli | 3.00 / bacon | Helium / oxygen | 125 W | 90 s | Open / 3 mm | Kim et al., 2011 |
| E. coli | 0.35 / pork loin | Helium / oxygen | 3 kV / 30 kHz | 10 min | Open / 3 mm | Kim et al., 2013 |
| E. coli | 2.54 / pork butt | Nitrogen / oxygen | 100 W / 15 kHz | 10 min | Closed / - | Jayasena et al., 2015 |
| E. coli | 2.57 / beef loin | Nitrogen / oxygen | 100 W / 15 kHz | 10 min | Closed / - | Jayasena et al., 2015 |
| E. coli | 2.65 / beef jerky | Ambient air | 15 kHz | 10 min | Closed / - | Yong et al., 2017a |
| S. Typhimurium | 1.7 / bacon | Helium / oxygen | 125 W | 90 s | Open / 3 mm | Kim et al., 2011 |
| S. Typhimurium | 2.68 / pork butt | Nitrogen / oxygen | 100 W / 15 kHz | 10 min | Closed / - | Jayasena et al., 2015 |
| S. Typhimurium | 2.28 / beef loin | Nitrogen / oxygen | 100 W / 15 kHz | 10 min | Closed / - | Jayasena et al., 2015 |
| S. Typhimurium | 3.03 / beef jerky | Ambient air | 15 kHz | 10 min | Closed / - | Yong et al., 2017a |
| S. enterica | 2.54 / chicken breast | Ambient air | 30 kV / 0.5 kHz | 3 min | Open / 2 mm | Dirks et al., 2012 |
| C. jejuni | 2.45 / chicken breast | Ambient air | 30 kV / 0.5 kHz | 3 min | Open / 2 mm | Dirks et al., 2012 |
Several factors influence the microbicidal efficacy of plasma treatment. APCP can be generated by DBD from nitrogen, oxygen, helium, and argon, as well as from ambient air. Of the ROS found in plasma, ozone is the major microbicidal species. Ozone concentrations in the gas phase discharge is related to the oxygen concentration of the discharge gas (Eliasson and Kogelschatz, 1991). Kovačević et al. (2017) showed that ozone is the dominant species produced in gas phase discharges composed of oxygen alone as compared to those with air, nitrogen, helium, or argon, and RNS are dominant in the gas phase discharges composed of air or nitrogen. Kim et al. (2011) found a large reduction in L. monocytogenes, E. coli, and S. Typhimurium levels in bacon when it was treated with plasma with a discharge gas consisting of helium and oxygen as compared to a discharge gas composed of helium alone. Furthermore, treatment with a mixture of nitrogen and oxygen was more effective in reducing the levels of L. monocytogenes in chicken breast than with nitrogen alone (Lee et al., 2011). Input power is another important factor in the microbicidal efficacy of plasma. Kim et al. (2011) found that the microbicidal effects of plasma treatment on L. monocytogenes, E. coli, and S. Typhimurium in inoculated bacon increased with an increase in input power from 75 to 125 W. Furthermore, Laroussi and Leipold (2004) found that an increase in the input power from 1.5 to 10 W increased the concentration of hydroxyl radicals and ozone in DBD plasma generated from atmospheric air.
Recently, the interest in In-package (closed) plasma treatment has increased. In-package plasma treatment is conducted using a flexible thin-layer electrode inside the sealed package and it has several benefits (Yong et al., 2017a). At first, subsequent contamination by microorganisms is prevented because the pasteurized food is transported in the sealed package to the consumer. Secondly, the long-lived reactive species, particularly ozone and hydrogen peroxide, generated in the sealed package pasteurize microorganisms continuously after plasma treatment (Yong et al., 2014).
Jayasena et al. (2015) reported 2.04, 2.54, and 2.68 Log CFU/g reductions in L. monocytogenes, E. coli, and S. Typhimurium levels, respectively, in inoculated pork shoulder after plasma treatment for 10 min in the package with a gas mixture of nitrogen and oxygen. Furthermore, L. monocytogenes, E. coli, and S. Typhimurium levels in beef jerky were reduced to as much as 2.36, 2.65, and 3.03 Log CFU/g, respectively, with In package plasma treatment for 10 min using ambient air (Yong et al., 2017a). However, previous studies have also reported that an increase in the discharge gas humidity from water vapor decreases ozone production (Kovačević et al. 2017; Ono and Oda, 2003). Kovačević et al. (2017) reported that plasma treatment heats the liquid, thereby increasing the humidity via the vaporization of water. Jung et al. (2017b) reported the temperature of meat batter increased from 0.2 to 10°C after plasma treatment for 30 min. Therefore, ozone production may be reduced when using In-package plasma treatment because the humidity in the package can be increased via the vaporization of moisture in samples.
Nitrite is a multifunctional curing additive used in the production of cured meat products. The addition of nitrite results in the development of a cured color and flavor (Parthasarathy and Bryan, 2012; Sebranek et al., 2012). Furthermore, it can prevent contamination by pathogenic microorganisms, including Clostridium botulinum (Jung et al., 2017b; Sebranek et al., 2012). Synthetic and natural nitrite sources, including sodium nitrite, potassium nitrite, and vegetable juice powder, are widely used in the meat product industry (Alahakoon et al., 2015; Parthasarathy and Bryan, 2012; Sebranek et al., 2012).
Several studies have shown that APCP treatment of liquids can result in the generation of nitrite (Ercan et al., 2016; Kojtari et al., 2013; Oehimigen et al., 2010). Plasma contains various RNS and nitrogen oxides, including NO2, NO3, N2O, N2O3, and N2O5, which are relatively stable species (Sakiyama et al., 2012). Nitrogen oxides in the gas-phase discharges diffuse and dissolve in liquids after plasma treatment. The dissolved nitrogen oxides then form nitric and nitrous acids via reactions with water molecules, and subsequently decompose into nitrate and nitrite, respectively (1-4) (Lukes et al, 2014; Rayson et al., 2012; Thomas and Vanderschuren, 2000).
The nitrite concentration in plasma-treated liquids decreases with the increase in plasma treatment time (Ercan et al., 2016; Kojtari et al., 2013; Oehimigen et al., 2010). The generation of nitrite is also accompanied by the release of hydrogen ions which can decrease the pH of the liquid from 7 to 2 (Jung et al., 2015). Nitrite is unstable under acidic conditions and forms nitrous acid. It subsequently decomposes into nitrate and nitrogen oxide (5-7) (Rayson et al., 2012; Thomas and Vanderschuren, 2000).
However, under alkaline conditions the nitrite generated by plasma persists (Jung et al., 2015; Lukes et al., 2014). Furthermore, Jung et al. (2015) showed that plasma treated water can contain up to 782 mg L−1 of nitrite when distilled water containing sodium pyrophosphate is exposed to APCP for 120 min.
The nitrite content of products treated with APCP is shown in Table 2. Emulsion sausages cured using plasma treated water containing nitrite had similar properties in terms of color, lipid oxidation, and sensory characteristics as those cured with sodium nitrite (Jung et al., 2015). Furthermore, Yong et al. (2017b) found that pork loin hams manufactured using brine injections consisting of plasma treated water or sodium nitrite had similar color, nitrosoheme pigment content, and lipid oxidation. However, the residual nitrite content of the emulsion sausage and pork loin ham cured with plasma treated water was lower than for those cured with sodium nitrite (Jung et al., 2015; Yong et al., 2017b). Natural nitrite sources are produced from vegetables containing nitrate which is subsequently converted into nitrite by nitrate reductase (Parthasarathy and Bryan, 2012). Therefore, vegetables that do not contain nitrate cannot be candidates of natural nitrite sources although it has high antioxidative and antimicrobial activities. However, natural nitrite has been derived from Perilla frutescens, which does not contain nitrate (Jung et al., 2017a). Jung et al. (2017a) found that the nitrite content of ethanolic extracts from P. frutescens increased following APCP treatment. Lyophilized P. frutescens powder extracts following APCP treatment contained nitrite at a concentration of 3.74 mg g−1, and exhibited increased antimicrobial activity against C. perfringens and S. Typhimurium when compared with those without APCP treatment (Jung et al., 2017a).
The direct curing effects of APCP treatment are shown in Table 2. APCP treatment of meat batter resulted in the generation of nitrite (Jung et al., 2017b; Lee et al., 2018) and the meat batter was found to contain 65.96 mg kg−1 nitrite after APCP treatment for 30 min and it developed a cured color after cooking (Jung et al., 2017a). Lee et al. (2018) found 42 mg kg−1 nitrite in meat batter cured in the developed plasma curing mixer treated with APCP for 30 min while mixing (Fig 2). Furthermore, canned ground ham manufactured from meat batter treated with APCP for 30 min showed no difference in color, residual nitrite content, texture, or sensorial properties as compared to ground ham cured at same nitrite level of 42 mg kg−1 by addition of sodium nitrite or celery powder (Lee et al., 2018).
| Substrate | Discharge gas | Power / Frequency | Exposure time | Nitrite content | Reference |
|---|---|---|---|---|---|
| Distilled water | Ambient air | 200 W / 15 kHz | 120 min | 782 mg L−1 | Jung et al., 2015 |
| Yong et al., 2017b | |||||
| Red perilla extract | Ambient air | 550 W / 25 kHz | 60 min | 3.74 g kg−1 | Jung et al., 2017a |
| Meat batter | Ambient air | 550 W / 25 kHz | 30 min | 65.96 mg kg−1 | Jung et al., 2017b |
| Meat batter | Ambient air | 600 W / 25 kHz | 30 min | 42 mg kg−1 | Lee et al., 2018 |
Considerations
The ROS found in plasma are important for the inhibition of microorganism growth. However, ROS, especially free radicals, can catalyze lipid and protein oxidation that can cause deterioration in the quality of meat and meat products (Armenteros et al. 2016; Lund et al., 2011). While some studies have found increased lipid oxidation in pork, beef, and bacon after APCP treatment (Jayasena et al., 2015; Kim et al., 2011), contrary findings have also been reported. Jung et al. (2017b) found no increase in lipid oxidation in meat batters after APCP treatment for 30 min. Furthermore, no differences in lipid and protein oxidation levels were found in ground hams cured by APCP treatment for 30 min, sodium nitrite, or celery powder at same nitrite level of 42 mg kg−1 (Lee et al., 2018). The temperature of APCP is similar to that of room temperature (Nehra et al., 2008). Jung et al. (2017b) reported an increase in the temperature of meat batter from 0.2 to 10°C during APCP treatment for 30 min, while Lee et al. (2018) found a temperature change from 1.0 to 8.9°C in meat batter under the same treatment conditions. Therefore, while APCP does raise the temperature of meat batter, this increase is small.
RNS in plasma generates nitrite in a treated substrate via reactions with water molecules (Jung et al., 2017b; Lee et al., 2018). Although Bauer et al. (2017) found that APCP treatment of beef loin did not increase nitrite levels, it is possible that APCP treatment of fresh meat forms nitrite, even though nitrite is usually only used for cured meat products. Previous studies have reported that the dominant reactive species in plasma are related to discharge gas composition. The dominant species are ROS in plasma generated with oxygen and RNS in plasma generated with air and nitrogen (Kovačević et al., 2017). Furthermore, an increase in input power results in an increase in RNS and a decrease in ozone concentrations (Bauer et al., 2017). Therefore, operating conditions must be taken into account depending on the objective of the APCP treatment.
Lee et al. (2016) reported no mutagenicity in chicken breast treated with APCP. Kim et al. (2016) found no mutagenicity in emulsion sausage cured by plasma treated water and no immune toxicity, based on tumor necrosis factor-α levels in mice fed emulsion sausage for 32 d. In addition, no mutagenicity in pork loin ham cured by plasma treated water has been reported (Yong et al., 2017b). However, there are limited data that fully confirm the safety of meat and meat products treated with APCP, therefore, further testing is necessary to satisfy consumers and the authorities.
Conclusion
APCP has been deemed to be an eco-friendly technology because it does not produce residues or toxic molecules. APCP treatment can effectively increase the shelf life and safety of meat and meat products via pasteurization of spoilage and pathogenic microorganisms without thermal damage. Furthermore, APCP is unique in that it can be used to produce nitrite and directly cure the meat products. It may seem obvious that the ROS, especially ozone, are important substances for the microbicidal effect of APCP treatment, and the RNS are main substances for the curing effect of APCP treatment. However, the generation of ROS and RNS in APCP can be influenced by several factors. Therefore, optimal operation conditions, including discharge gas composition, input power, and type (open or closed), must be optimized in accordance with the objective of the APCP treatment. In addition, the present studies of APCP treatment in meat and meat products have been conducted at the laboratory scale. Therefore, the efficiency of APCP treatment has to be evaluated at the industrial scale for the increase in the industrial applicability. Nonetheless, we conclude that APCP is a promising technology for use in the meat and meat product industry as a non-thermal pasteurization and curing methods.